PROBLEMS
PROBLEMS
Question 23.1
Getting exposure. Proteins are denatured by acid in the stomach. This denaturation makes them better substrates for proteolysis. Explain why this is the case.
Question 23.2
Targeting for destruction. What are the steps required to attach ubiquitin to a target protein?
Question 23.3
Not the dating service. Match the description on the right with the term on the left.
|
(a) Pepsin ______ (b) N- (c) Ubiquitin ______ (d) PEST sequence ______ (e) Threonine nucleophiles ______ (f) ATP- (g) Proteasome ______ (h) Ubiquitin- (i) Ubiquitin- (j) Ubiquitin- |
1. Requires an adenylate intermediate 2. Marks a protein for destruction 3. 19S regulatory subunit 4. Determines half- 5. 20S core 6. Substrate for ligase 7. Stomach proteolytic enzyme 8. Recognizes protein to be degraded 9. Protein degrading machine 10. Pro- |
Question 23.4
Wasted energy? Protein hydrolysis is an exergonic process, yet the 26S proteasome is dependent on ATP hydrolysis for activity.
Explain why ATP hydrolysis is required by the 26S proteasome.
Small peptides can be hydrolyzed without the expenditure of ATP. How does this information concur with your answer to part a?
Question 23.5
Keto counterparts. Name the α-ketoacid that is formed by the transamination of each of the following amino acids:
Alanine
Aspartate
Glutamate
Leucine
Phenylalanine
Tyrosine
Question 23.6
A versatile building block.
Write a balanced equation for the conversion of aspartate into glucose through the intermediate oxaloacetate. Which coenzymes participate in this transformation?
Write a balanced equation for the conversion of aspartate into oxaloacetate through the intermediate fumarate.
Question 23.7
The benefits of specialization. The archaeal proteasome contains 14 identical active β subunits, whereas the eukaryotic proteasome has 7 distinct β subunits. What are the potential benefits of having several distinct active subunits?
Question 23.8
Propose a structure. The 19S subunit of the proteasome contains six subunits that are members of the AAA ATPase family. Other members of this large family are associated into homohexamers with sixfold symmetry. Propose a structure for the AAA ATPases within the 19S proteasome. How might you test and refine your prediction?
709
Question 23.9
Effective electron sinks. Pyridoxal phosphate stabilizes carbanionic intermediates by serving as an electron sink. Which other prosthetic group catalyzes reactions in this way?
Question 23.10
Cooperation. How do aminotransferases and glutamate dehydrogenase cooperate in the metabolism of the amino group of amino acids?
Question 23.11
Taking away the nitrogen. What amino acids yield citric acid cycle components and glycolysis intermediates when deaminated?
Question 23.12
One reaction only. What amino acids can be deaminated directly?
Question 23.13
Useful products. What are the common features of the breakdown products of the carbon skeletons of amino acids?
Question 23.14
Helping hand. Propose a role for the positively charged guanidinium nitrogen atom in the cleavage of argininosuccinate into arginine and fumarate.
Question 23.15
Nitrogen sources. What are the immediate biochemical sources for the two nitrogen atoms in urea?
Question 23.16
Counterparts. Match the biochemical on the right with the property on the left.
|
(a) Formed from NH4+ ______ (b) Hydrolyzed to yield urea ______ (c) A second source of nitrogen ______ (d) Reacts with aspartate ______ (e) Cleavage yields fumarate ______ (f) Accepts the first nitrogen ______ (g) Final product ______ |
1. Aspartate 2. Urea 3. Ornithine 4. Carbamoyl phosphate 5. Arginine 6. Citrulline 7. Arginosuccinate |
Question 23.17
Line up. Identify structures A–
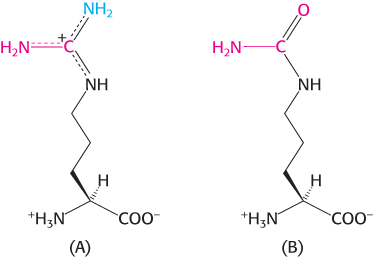
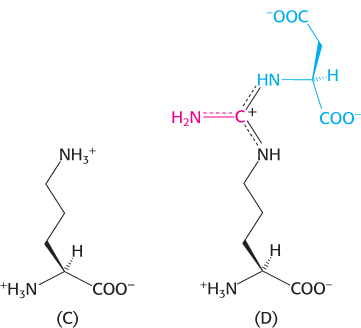
Question 23.18
Completing the cycle. Four high-
Question 23.19
A good bet. A friend bets you a bazillion dollars that you can’t prove that the urea cycle is linked to the citric acid cycle and other metabolic pathways. Can you collect?
Question 23.20
Inhibitor design. Compound A has been synthesized as a potential inhibitor of a urea-
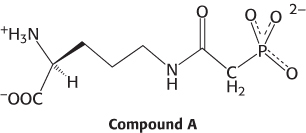
Question 23.21
Ammonia toxicity. Glutamate is an important neurotransmitter whose levels must be carefully regulated in the brain. Explain how a high concentration of ammonia might disrupt this regulation. How might a high concentration of ammonia alter the citric acid cycle?
Question 23.22
A precise diagnosis. The urine of an infant gives a positive reaction with 2,4-
Question 23.23
Therapeutic design. How would you treat an infant who is deficient in argininosuccinate synthetase? Which molecules would carry nitrogen out of the body?
Question 23.24
Damaged liver. As we will see later (Chapter 27), liver damage (cirrhosis) often results in ammonia poisoning. Explain why this is the case.
710
Question 23.25
Argininosuccinic aciduria. Argininosuccinic aciduria is a condition that results when the urea-
Question 23.26
Sweet hazard. Why should phenylketonurics avoid using aspartame, an artificial sweetener? (Hint: Aspartame is l-aspartyl-
Question 23.27
Déjà vu. N-Acetylglutamate is required as a cofactor in the synthesis of carbamoyl phosphate. How is N-acetylglutamate synthesized from glutamate?
Question 23.28
Negative nitrogen balance. A deficiency of even one amino acid results in a negative nitrogen balance. In this state, more protein is degraded than is synthesized, and so more nitrogen is excreted than is ingested. Why would protein be degraded if one amino acid were missing?
Question 23.29
Precursors. Differentiate between ketogenic amino acids and glucogenic amino acids.
Question 23.30
A slight of hand. The end products of tryptophan degradation are acetyl CoA and acetoacetyl CoA, yet tryptophan is a gluconeogenic amino acid in animals. Explain.
Question 23.31
Closely related. Pyruvate dehydrogenase complex and α-ketoglutarate dehydrogenase complex are huge enzymes consisting of three discrete enzymatic activities. Which amino acids require a related enzyme complex, and what is the name of the enzyme?
Question 23.32
Supply lines. The carbon skeletons of the 20 common amino acids can be degraded into a limited number of end products. What are the end products and in what metabolic pathway are they commonly found?
Mechanism Problems
Question 23.33
Serine dehydratase. Write out a complete mechanism for the conversion of serine into aminoacrylate catalyzed by serine dehydratase.
Question 23.34
Serine racemase. The nervous system contains a substantial amount of d-serine, which is generated from l-serine by serine racemase, a PLP-
Chapter Integration Problems
Question 23.35
Multiple substrates. In Chapter 8, we learned that there are two types of bisubstrate reactions, sequential and double-
Question 23.36
Double duty. Degradation signals are commonly located in protein regions that also facilitate protein–
Question 23.37
Fuel choice. Within a few days after a fast begins, nitrogen excretion accelerates to a higher-
What events trigger the initial surge of nitrogen excretion?
Why does nitrogen excretion fall after several weeks of fasting?
Explain the increase in nitrogen excretion when the lipid stores have been depleted.
Question 23.38
A serious situation. Pyruvate carboxylase deficiency is a fatal disorder. Patients with pyruvate carboxylase deficiency sometimes display some or all of the following symptoms: lactic acidosis, hyperammonemia (excess NH4+ in the blood), hypoglycemia, and demyelination of the regions of the brain due to insufficient lipid synthesis. Provide a possible biochemical rationale for each of these observations.
Question 23.39
Isoleucine degradation. Isoleucine is degraded to acetyl CoA and succinyl CoA. Suggest a plausible reaction sequence, based on reactions discussed in the text, for this degradation pathway.
Question 23.40
Many roles. Pyridoxal phosphate is an important coenzyme in transamination reactions. We have seen this coenzyme before, in glycogen metabolism. Which enzyme in glycogen metabolism requires pyridoxal phosphate and what role does the coenzyme play in this enzyme?
Question 23.41
Enough cycles to have a race. The glucose–
Data Interpretation Problem
Question 23.42
Another helping hand. In eukaryotes, the 20S proteasome component in conjunction with the 19S component degrades ubiquitinated proteins with the hydrolysis of a molecule of ATP. Archaea lack ubiquitin and the 26S proteasome but do contain a 20S proteasome. Some archaea also contain an ATPase that is homologous to the ATPases of the eukaryotic 19S component. This archaeal ATPase activity was isolated as a 650-
711
Protein degradation was measured as a function of time and in the presence of various combinations of components. Graph A shows the results.
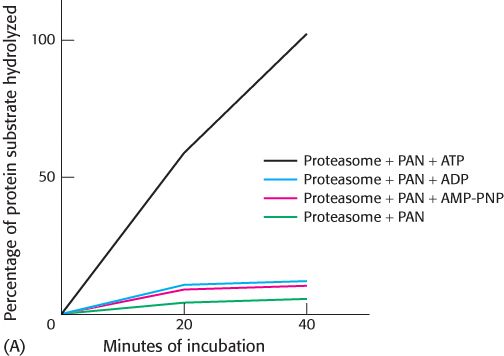
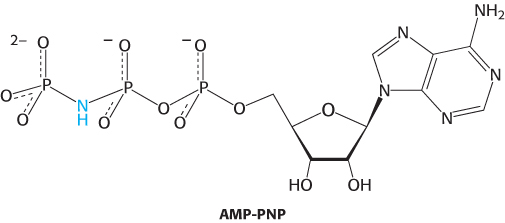
What is the effect of PAN on archaeal proteasome activity in the absence of nucleotides?
What is the nucleotide requirement for protein digestion?
What evidence suggests that ATP hydrolysis, and not just the presence of ATP, is required for digestion?
A similar experiment was performed with a small peptide as a substrate for the proteasome instead of a protein. The results obtained are shown in graph B.
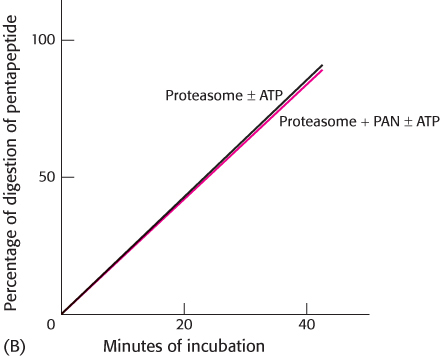
How do the requirements for peptide digestion differ from those of protein digestion?
Suggest some reasons for the difference.
The ability of PAN from the archaeon Thermoplasma to support protein degradation by the 20S proteasomes from the archaeon Methanosarcina and rabbit muscle was then examined.
Percentage of digestion of protein substrate
(20S proteasome source)
Additions
Thermoplasma
Methanosarcina
Rabbit muscle
None
11
10
10
PAN
8
8
8
PAN + ATP
100
40
30
PAN + ADP
12
9
10
[Data from P. Zwickl, D. Ng, K. M. Woo, H.-P. Klenk, and A. L. Goldberg. An archaebacterial ATPase, homologous to ATPase in the eukaryotic 26S proteasome, activates protein breakdown by 20S proteasomes. J. Biol. Chem. 274(1999): 26008–
26014.] Can the Thermoplasma PAN augment protein digestion by the proteasomes from other organisms?
What is the significance of the stimulation of rabbit muscle proteasome by Thermoplasma PAN?
712