30.5A Variety of Antibiotics and Toxins Can Inhibit Protein Synthesis
A Variety of Antibiotics and Toxins Can Inhibit Protein Synthesis
Many chemicals that inhibit various aspects of protein synthesis have been identified. These chemicals are powerful experimental tools and clinically useful drugs.
914
|
Antibiotic |
Action |
|---|---|
|
Streptomycin and other aminoglycosides |
Inhibit initiation and cause the misreading of mRNA (bacteria) |
|
Tetracycline |
Binds to the 30S subunit and inhibits the binding of aminoacyl- |
|
Chloramphenicol |
Inhibits the peptidyl transferase activity of the 50S ribosomal subunit (bacteria) |
|
Cycloheximide |
Inhibits translocation (eukaryotes) |
|
Erythromycin |
Binds to the 50S subunit and inhibits translocation (bacteria) |
|
Puromycin |
Causes premature chain termination by acting as an analog of aminoacyl- |
Some antibiotics inhibit protein synthesis
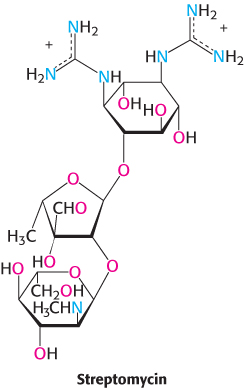
 The differences between eukaryotic and bacterial ribosomes can be exploited for the development of antibiotics (Table 30.4). For example, the antibiotic streptomycin, a highly basic trisaccharide, interferes with the binding of fMet-
The differences between eukaryotic and bacterial ribosomes can be exploited for the development of antibiotics (Table 30.4). For example, the antibiotic streptomycin, a highly basic trisaccharide, interferes with the binding of fMet-
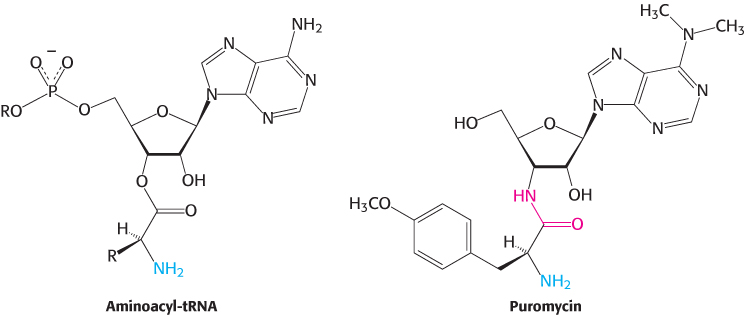
The antibiotic puromycin inhibits protein synthesis in both bacteria and eukaryotes by causing nascent polypeptide chains to be released before their synthesis is completed. Puromycin is an analog of the terminal part of aminoacyl-
Diphtheria toxin blocks protein synthesis in eukaryotes by inhibiting translocation
 Many antibiotics, harvested from bacteria for medicinal purposes, are inhibitors of bacterial protein synthesis. However, some bacteria produce protein-
Many antibiotics, harvested from bacteria for medicinal purposes, are inhibitors of bacterial protein synthesis. However, some bacteria produce protein-
915
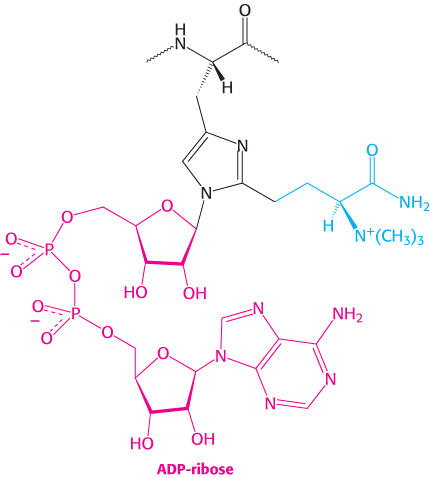
A single A fragment of the toxin in the cytoplasm can kill a cell. Why is it so lethal? EF2 contains diphthamide, an unusual amino acid residue that enhances the fidelity of codon shifting during translocation. Diphthamide is formed by a highly conserved complicated pathway that posttranslationally modifies histidine. The A fragment of the diphtheria toxin catalyzes the transfer of the ADP ribose unit of NAD+ to the diphthamide ring (Figure 30.31). This ADP ribosylation of a single side chain of EF2 blocks EF2′s capacity to carry out the translocation of the growing polypeptide chain. Protein synthesis ceases, accounting for the remarkable toxicity of diphtheria toxin.
Ricin fatally modifies 28S ribosomal RNA
 Ricin is a small protein (65 kDa) found in the seeds of the castor oil plant, Ricinus communis (Figure 30.32). It is indeed a deadly molecule because as little as 500 μg is lethal for an adult human being, and a single molecule can inhibit all protein synthesis in a cell, resulting in cell death.
Ricin is a small protein (65 kDa) found in the seeds of the castor oil plant, Ricinus communis (Figure 30.32). It is indeed a deadly molecule because as little as 500 μg is lethal for an adult human being, and a single molecule can inhibit all protein synthesis in a cell, resulting in cell death.

On September 7, 1978, an agent of the Soviet Union State Security Department (KGB), using a weapon built into an umbrella, embedded a small pellet containing ricin into the thigh of Bulgarian dissident Georgi Markov as he crossed Waterloo Bridge in London. Mr. Markov felt a sharp sting, as if from a bug bite. He died four days later.
Ricin is a heterodimeric protein composed of a catalytic A chain joined by a single disulfide bond to a B chain. The B chain allows the toxin to bind to the target cell, and this binding leads to an endocytotic uptake of the dimer and the eventual release of the A chain into the cytoplasm. The A chain, an N-