31.3Regulatory Circuits Can Result in Switching Between Patterns of Gene Expression
Regulatory Circuits Can Result in Switching Between Patterns of Gene Expression
The study of viruses that infect bacteria has led to significant advances in our understanding of the processes that control gene expression. Again, sequence-
The λ repressor regulates its own expression
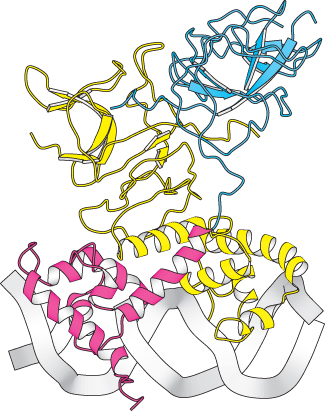
 FIGURE 31.14 Structure of the λ repressor bound to DNA. The λ repressor binds to DNA as a dimer. The amino-
FIGURE 31.14 Structure of the λ repressor bound to DNA. The λ repressor binds to DNA as a dimer. The amino-The first protein that we shall consider is the λ repressor, sometimes known as the λ cI protein. This protein is key because it blocks, either directly or indirectly, the transcription of almost all genes encoded by the virus. The one exception is the gene that encodes the λ repressor itself. The λ repressor consists of an amino-
933
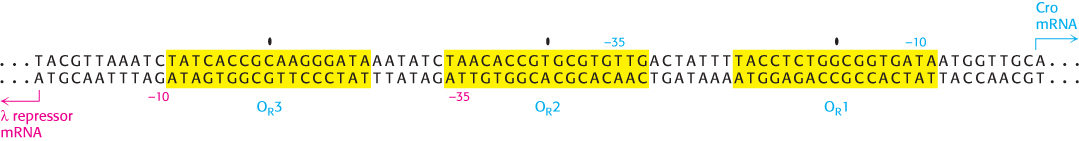
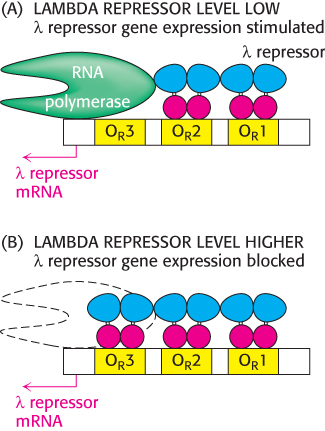
The λ repressor does not have the same affinity for the three sites; it binds the site OR1 with the highest affinity. In addition, the binding to adjacent sites is cooperative so that, after a λ repressor dimer has bound at OR1, the likelihood that a protein will bind to the adjacent site OR2 increases by approximately 25-
Thus, the λ repressor stimulates its own production. As the concentration of the λ repressor increases further, an additional repressor dimer can bind to the OR3 site, blocking the other promoter and repressing the production of additional repressor. Thus, the right operator serves to maintain the λ repressor in a narrow, stable concentration range (Figure 31.16). The λ repressor also blocks other promoters in the λ phage genome so that the repressor is the only phage protein produced, which corresponds to the lysogenic state.
A circuit based on the λ repressor and Cro forms a genetic switch
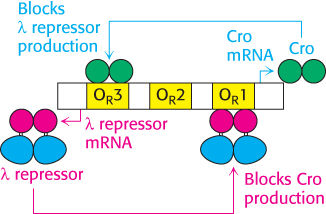
What stimulates the switch to the lytic pathway? Changes such as DNA damage initiate the cleavage of the λ repressor at a specific bond between the DNA-
Many prokaryotic cells release chemical signals that regulate gene expression in other cells
Prokaryotic cells have been traditionally viewed as solitary single cells. However, it is becoming increasingly clear that, in many circumstances, prokaryotic cells live in complex communities, interacting with other cells of their own and different species. These social interactions change the patterns of gene expression within the cells.
934
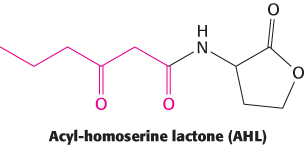
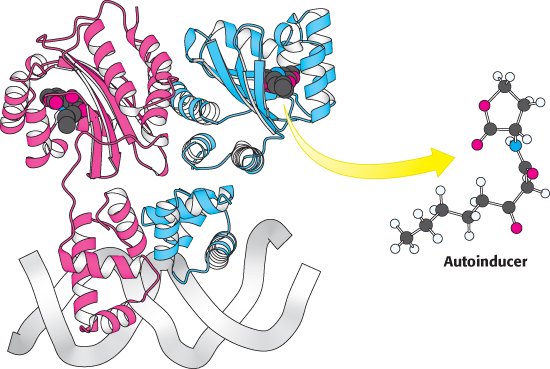
An important type of interaction is called quorum sensing. This phenomenon was discovered in the bacteria Vibrio fischeri, a species of bacterium that can live inside a specialized light organ in the bobtail squid. In this symbiotic relation, the bacteria produce luciferase and bioluminesce, providing protection for the squid (by preventing being backlit by moonlight) in exchange for a safer place to live and reproduce. When these bacteria are grown in culture at low density, they are not bioluminescent. However, when the cell density reaches a critical level, the gene for luciferase is expressed and the cells bioluminesce. A key observation was that, when V. fischeri cells were transferred to a sterile medium in which other V. fischeri cells had been grown to high density, the cells became bioluminescent even at low cell density. This experiment revealed that a chemical, subsequently shown to be N-3-
Cells of V. fischeri release the autoinducer into their environment and other V. fischeri cells take up the chemical. V. fischeri cells express a DNA-
Because each cell produces only a small amount of the autoinducer, this regulatory system allows each V. fischeri cell to determine the density of the V. fischeri population in its environment—
Biofilms are complex communities of prokaryotes
Many species of prokaryotes can be found in specialized structures termed biofilms that can form on surfaces. Biofilms are of considerable medical importance because organisms within them are often quite resistant to the immune response of the host as well as to antibiotics. Quorum sensing appears to play a major role in the formation of biofilms, in that cells are able to sense other cells in their environments and to promote the formation of communities with particular compositions. Some genes controlled by quorum-
935