34.2Antibodies Bind Specific Molecules Through Hypervariable Loops
Antibodies Bind Specific Molecules Through Hypervariable Loops
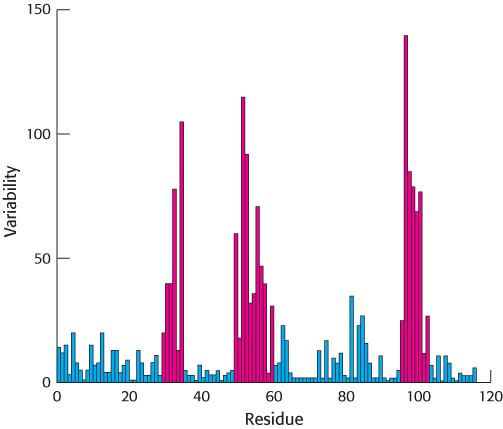
A comparison of the amino acid sequences of different IgG antibodies from human beings or mice shows that the carboxyl-
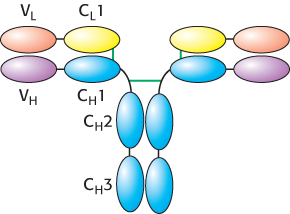
The immunoglobulin fold consists of a beta-sandwich framework with hypervariable loops
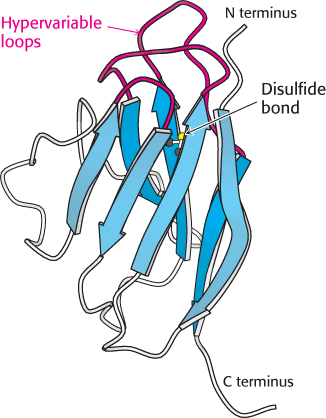
 FIGURE 34.12 Immunoglobulin fold. An immunoglobulin domain consists of a pair of β sheets linked by a disulfide bond and hydrophobic interactions. Notice that three hypervariable loops lie at one end of the structure.
FIGURE 34.12 Immunoglobulin fold. An immunoglobulin domain consists of a pair of β sheets linked by a disulfide bond and hydrophobic interactions. Notice that three hypervariable loops lie at one end of the structure.
An IgG molecule consists of a total of 12 immunoglobulin domains. These domains have many sequence features in common and adopt a common structure, the immunoglobulin fold (Figure 34.12). Remarkably, this same structural domain is found in many other proteins that play key roles in both immune and nonimmune functions.
The immunoglobulin fold consists of a pair of β sheets, each built of antiparallel β strands, that surround a central hydrophobic core. A single disulfide bond bridges the two sheets. Two aspects of this structure are particularly important for its function. First, three loops present at one end of the structure form a potential binding surface. These loops contain the hypervariable sequences present in antibodies and in T-
 The immunoglobulin fold is one of the most prevalent domains encoded by the human genome: more than 750 genes encode proteins with at least one immunoglobulin fold recognizable at the level of amino acid sequence. Such domains are also common in other multicellular animals such as flies and nematodes. However, from inspection of amino acid sequence alone, immunoglobulin-
The immunoglobulin fold is one of the most prevalent domains encoded by the human genome: more than 750 genes encode proteins with at least one immunoglobulin fold recognizable at the level of amino acid sequence. Such domains are also common in other multicellular animals such as flies and nematodes. However, from inspection of amino acid sequence alone, immunoglobulin-
989
X-ray analyses have revealed how antibodies bind antigens
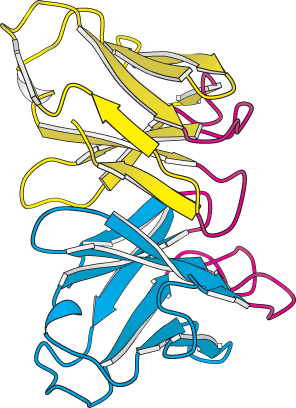
 FIGURE 34.13 Variable domains. A side view of the variable domains of the L chain (yellow) and the H chain (blue); the complementarity-
FIGURE 34.13 Variable domains. A side view of the variable domains of the L chain (yellow) and the H chain (blue); the complementarity-For each class of antibody, the variable domains at the amino-
The results of x-
A few aspects of antibody binding merit specific attention, inasmuch as they relate directly to the structure of immunoglobulins. The binding site on the antibody incorporates some or all of the CDRs in the variable domains of the antibody. Small molecules are likely to make contact with fewer CDRs, with perhaps 15 residues of the antibody participating in the binding interaction. Macromolecules often make more extensive contact, sometimes interacting with all six CDRs and 20 or more residues of the antibody. Small molecules often bind in a cleft of the antigen-
A well-
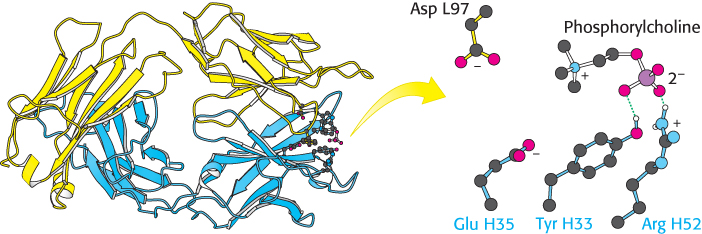
990
Residues from five CDRs participate in the binding of phosphorylcholine to human Fab. This binding does not significantly change the structure of the antibody, yet induced fit plays a role in the formation of many antibody–
Large antigens bind antibodies with numerous interactions
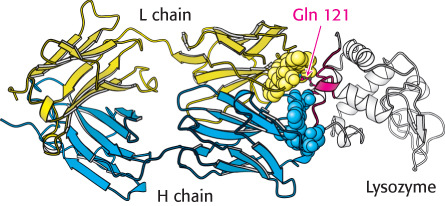
 FIGURE 34.16 Antibody–
FIGURE 34.16 Antibody–How do large antigens interact with antibodies? A large collection of antibodies against hen egg-
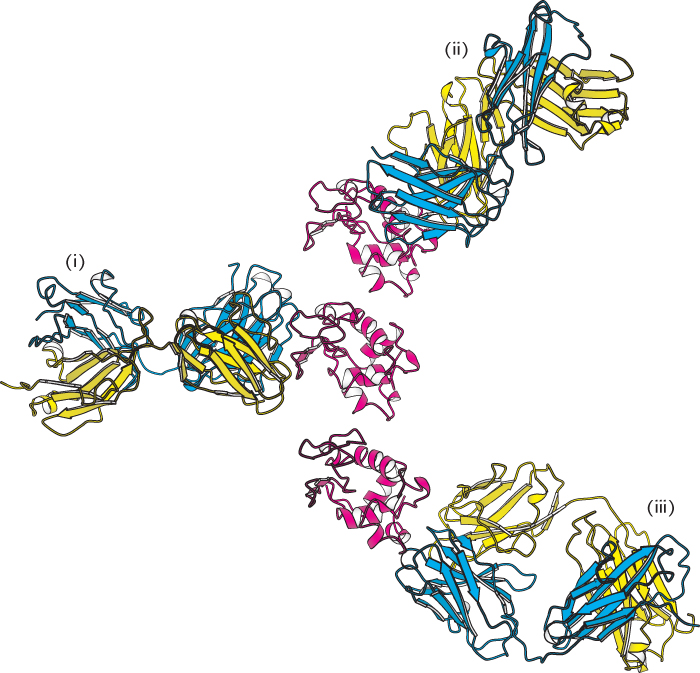
 FIGURE 34.15 Antibodies against lysozyme. The structures of three complexes (i, ii, iii) between Fab fragments (blue and yellow) and hen egg-
FIGURE 34.15 Antibodies against lysozyme. The structures of three complexes (i, ii, iii) between Fab fragments (blue and yellow) and hen egg-All six CDRs of the antibody make contact with this epitope. The region of contact is quite extensive (about 30 × 20 Å). The apposed surfaces are rather flat. The only exception is the side chain of glutamine 121 of lysozyme, which penetrates deeply into the antibody’s binding site, where it forms a hydrogen bond with a main-
991