3.4 STEREOCHEMISTRY
The concept of stereochemistry, the spatial arrangement of the atoms within a molecule, is critical for understanding the structures and activities of biological molecules. As we shall see, the orientation of the chemical bonds of nucleic acids and proteins influences how these molecules fold in three dimensions, bind to other molecules, and catalyze reactions.
Three-Dimensional Atomic Arrangements Define Molecules
Our understanding of stereochemistry stems from a discovery by the French physicist Dominique Arago in 1811. Arago found that plane-polarized light, which vibrates in just one plane, rotates when it is sent through a piece of quartz crystal. Other scientists subsequently showed that many, but not all, substances share the ability to rotate the plane of plane-polarized light; a substance with this ability is said to be optically active. In 1848, 26-year-old chemist and crystallographer Louis Pasteur proposed that if a molecule is not superposable on its mirror image, it will be optically active; otherwise, it is optically inactive. This bold prediction subsequently proved to be correct. In fact, all objects can be classified as those that can be superposed on their mirror image, such as golf balls and champagne glasses, and those that cannot, such as hands. Objects that can be superposed on their mirror image are said to be achiral; those that cannot are chiral (Figure 3-22). All optically active chemical compounds are chiral, and all optically inactive compounds are achiral.
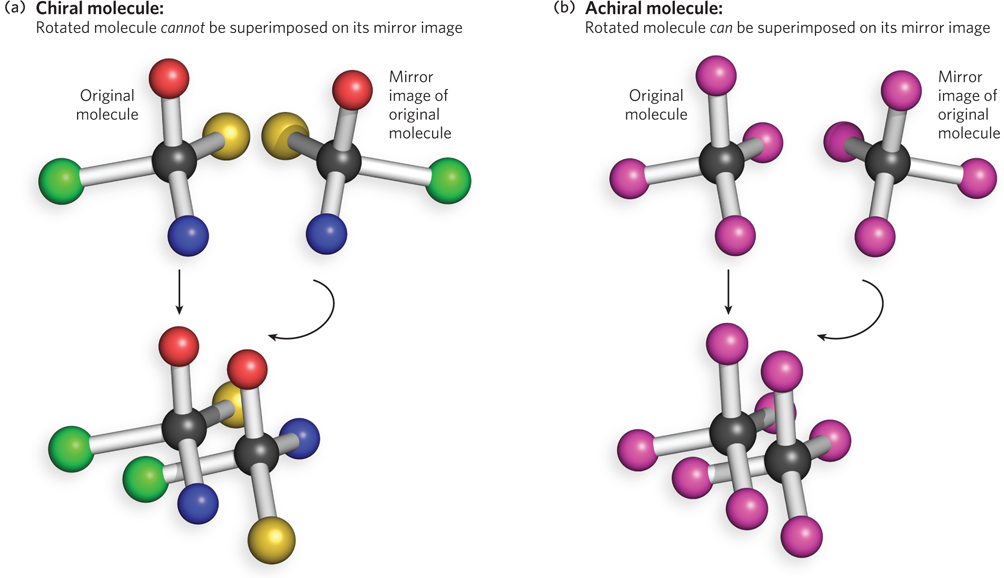
Figure 3-22: Chiral versus achiral molecules. (a) A molecule is chiral if it is not identical to its mirror image (i.e., it cannot be superposed on its image). When bonded to four atoms or functional groups that are not identical to each other, a carbon atom is chiral, because the four atoms or functional groups can be arranged in two different ways that are not superposable mirror images. All four of the bonded groups must be different from each other for the carbon center to be chiral. Thus, the two forms of the molecule have the same chemical formula but different chemical behavior. (b) In contrast, a carbon atom bonded to four identical atoms is achiral, because only one configuration is possible and any arrangement of the four bound atoms is superposable on its mirror image.
This is an important idea in molecular biology, because many biologically relevant molecules are chiral. For example, any carbon atom connected to four different groups—such as the α-carbon atom in an amino acid or certain carbons in the pentose sugars of nucleotides—is an asymmetric carbon atom, or chiral center. Thus, all nucleic acids and proteins are chiral, because they contain carbon atoms that are chiral centers, as we shall see shortly.
KEY CONVENTION
Two different arrangements of atoms in a molecule that cannot be interconverted without breaking and re-forming one or more covalent bonds are said to have different configurations. Two different arrangements of atoms that can be interconverted without breaking and re-forming a covalent bond, such as by rotation about a single bond, are said to have different conformations.
Biological Molecules and Processes Selectively Use One Stereoisomer
Two molecules that have the same chemical and structural formula but differ in the arrangement of their atoms in space (i.e., are not superposable) are called stereoisomers. A pair of stereoisomers that are also mirror images of each other are called enantiomers. All physical and chemical properties of enantiomers are identical, except for two. First, the two enantiomers rotate the plane of plane-polarized light in opposite directions. Second, two enantiomers react at the same rate with any achiral compound but at different rates with any chiral compound.
For particular reference molecules selected by early chemists, including Pasteur, the one that rotates light to the right is called the (+) or d form (dextrorotatory), and the one that rotates light to the left is called the (−) or l form (levorotatory). Note that these “d” and “l” prefixes are distinct from the “D” and “L” prefixes (in small capitals) that refer to the actual configuration of each enantiomer (regardless of their light-rotating properties). Many chiral biological molecules are defined as D or L configurations based on their chemical relationship to the two forms of a reference compound, whether or not a pure solution of one form of that molecule is levorotatory or dextrorotatory. For amino acids, the reference compound is glyceraldehyde, and the version synthesized from (+) glyceraldehyde is considered to be the D form. Nine of the nineteen L-amino acids commonly found in proteins (glycine is achiral), however, are actually dextrorotatory!
Many biological molecules are chiral, and living organisms usually use only one of the two possible enantiomers. Thus, biochemical reactions have evolved to recognize and favor one stereoisomer over the other. For example, the simple sugar glucose is chiral. D-Glucose can be broken down by digestive enzymes, which are also chiral, at a rate commensurate with its use as an energy source. Because digestive enzymes are chiral, they do not recognize or digest glucose that has the opposite chirality (L-glucose), even though the two sugar molecules are identical in chemical and structural formulas.
Proteins and Nucleic Acids Are Chiral
Proteins are chiral because nearly all of the common amino acids contain an α carbon bonded to four different groups: a carboxyl group, an amino group, an R group, and a hydrogen atom. (The amino acid glycine is the exception, because its R group is simply a hydrogen atom.) The α carbon is therefore a chiral center, and the tetrahedral arrangement of the bonding orbitals around the α-carbon atom enables the four different functional groups to occupy two different possible spatial arrangements. This means that each amino acid, except glycine, has two possible stereoisomers. Because these are nonsuperposable mirror images of each other, the two forms are enantiomers (Figure 3-23a). Like all molecules with a chiral center, amino acids are optically active and rotate plane-polarized light. Nature uses only one of these enantiomeric forms in proteins, and it is the same one for each amino acid—the L form. D-Amino acids are almost never found in nature—a phenomenon that has been cleverly harnessed by researchers to design new therapeutics (Highlight 3-1).
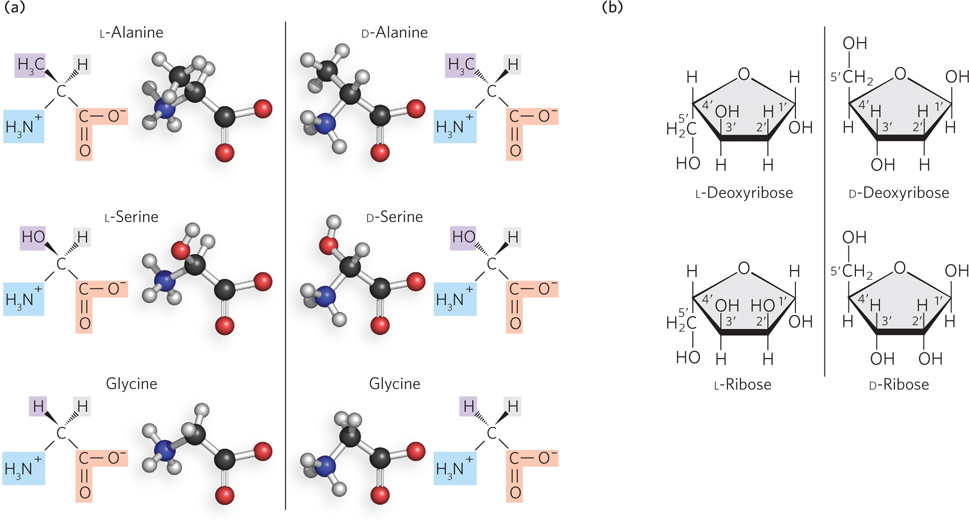
Figure 3-23: Enantiomers of amino acids and nucleotides. (a) Each amino acid, except glycine, has two possible stereoisomers. These nonsuperposable mirror images (enantiomers) are known as L and D forms. Only the L forms of amino acids are found in natural proteins. (b) All of the carbon atoms in ribose except C-5′ and all in deoxyribose except C-2′ and C-5′ are chiral centers. Nature uses only the D-enantiomeric form of the sugars—D-deoxyribose or D-ribose—as the building blocks for DNA and RNA.
Nucleic acids, too, are chiral molecules. Nucleotides contain several chiral centers in the ribose or deoxyribose ring—all of the carbon atoms in ribose except for C-5′ and all carbons in deoxyribose except for C-2′ or C-5′ are asymmetric (Figure 3-23b). Again, nature uses one enantiomeric form of the sugar—D-ribose or D-deoxyribose—in the building blocks for RNA and DNA. Enzymes that act on DNA or RNA have evolved to recognize this chiral arrangement of the nucleic acids.
SECTION 3.4 SUMMARY
A molecule with a structure that cannot be superposed on its mirror image is chiral.
All nucleic acids and proteins are chiral, because they contain carbon atoms bonded to four different atoms or groups. Because the four bonds of carbon point to the four corners of a tetrahedron, and the four functional groups can be spatially arranged in two different ways, these carbon atoms are chiral centers.
Stereoisomers are pairs of molecules that have the same chemical and structural formulas but are not superposable on each other, because they differ in the spatial arrangement of their atoms. Biochemical reactions have evolved to recognize and favor one stereoisomer over the other.
Enantiomers are pairs of stereoisomers that are mirror images of each other. Enantiomers rotate the plane of plane-polarized light in opposite directions. The D and L forms of major biological molecules are defined not by the direction in which they rotate plane-polarized light but by their structural relationship to a reference compound—glyceraldehyde in the case of α-amino acids.
-
Only the L enantiomers of amino acids are found in natural proteins. Like all molecules with a chiral center, amino acids are optically active and rotate plane-polarized light.
Only the D enantiomer of the pentose sugar—D-ribose or D-deoxyribose—is used in the building blocks for RNA and DNA.
HIGHLIGHT 3-1 MEDICINE: The Behavior of a Peptide Made of D-Amino Acids
L-Peptides capable of binding and blocking the function of important therapeutic targets—such as viral enzymes or receptor proteins—have turned out to be virtually useless for treating patients, because once introduced into the body, they are quickly destroyed. This is because cells and blood serum contain proteases, enzymes that bind to normal, L-amino acid–containing peptides and catalyze their rapid degradation. To get around this problem, Peter Kim, then an MIT professor and Howard Hughes Medical Institute investigator, wondered whether unnatural peptides made of D-amino acids would be useful as therapeutics in cells or serum.
Kim and his coworkers synthesized a portion of CD8, the human immunodeficiency virus (HIV) protein required for infection, in the unnatural, D-amino acid form, and they used it to identify L-amino acid peptides that bind specifically to this protein (Figure 1, left). These L-amino acid peptides were then resynthesized, but this time from D-amino acids. Because D and L-amino acids are mirror images of each other, the new D-amino acid peptides bound to the target CD8 protein of the natural (L) form (Figure 1, right). Thus, the enantiomeric D-amino acid peptides could be used therapeutically to block the activity of the natural viral protein, while avoiding degradation by human enzymes that act only on natural peptides. This clever approach was subsequently used to make D-peptide–based drugs that were tested in clinical trials for inhibiting the entry of HIV into cells. Although these peptides were not effective as HIV therapeutics, in part due to difficulties in getting them into infected cells, the strategy of using biologically inert enantiomers as drugs remains attractive.
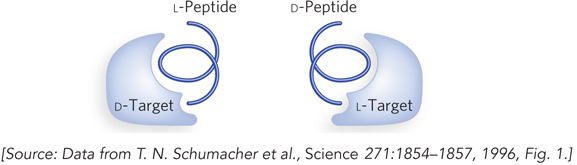
FIGURE 1 Kim’s experiment to isolate L-amino acid peptides that bind to an unnatural target protein and then synthesize D-peptides that will bind to the natural protein. Because of the mirror-image relationship between L and D-amino acids, L-peptides that bind to the D form of a target protein have the same sequence as D-peptides that bind to the L form of the target protein.


