PROBLEMS
Question 4.1
Each ionizable group of an amino acid can exist in one of two states, charged or neutral. The electric charge on the functional group is determined by the relationship between the group’s pKa and the pH of the solution. This relationship is described by the Henderson-
Histidine has three ionizable groups. Write the equilibrium equations for its three ionizations and assign the proper pKa for each. Draw the structure of histidine in each ionization state. What is the net charge on the histidine molecule in each ionization state?
Draw the structures of the predominant ionization states of histidine at pH 1, 4, 8, and 12. Note that the ionization state can be approximated by treating each ionizable group independently.
What is the net charge of histidine at pH 1, 4, 8, and 12? For each pH, will histidine migrate toward the anode (+) or cathode (−) when placed in an electric field?
Question 4.2
A quantitative amino acid analysis reveals that bovine serum albumin (BSA) contains 0.58% tryptophan (Mr 204) by weight.
Calculate the minimum molecular weight of BSA (i.e., assume there is only one Trp residue per protein molecule).
The BSA protein is purified and its molecular weight is estimated to be 70,000. How many Trp residues are present in a molecule of serum albumin?
Question 4.3
A peptide has the following sequence:
E–
What is the net charge of the molecule at pH 3, 8, and 11? (Use pKa values for side chains and terminal amino and carboxyl groups as given in Table 4-1.)
Purification of a peptide or protein is often easier if you understand its ionization properties. At a pH called the isoelectric point (pI), the net charge of the peptide or protein is zero. At lower or higher pH, it has a net positive or net negative charge, respectively. Estimate the pI for the above peptide.
Question 4.4
A biochemist isolates a peptide hormone with the following sequence:
ADSERNCQLVILLAWLPGVKVQCALLDRET
Which of these residues could contribute a positive charge? (Assume the residues are numbered 1 through 30, left to right.)
Which residues contribute a negative charge?
Which residues can be connected by a disulfide bond?
Question 4.5
The sequence shown below, with 86 amino acid residues, folds into a β sheet substructure within a protein. The residues that form the β strands are noted (by residue number) above the sequence, and those outside the sheet are shown below. What type of β sheet structure is likely to form, parallel or antiparallel? Explain. What types of secondary structure are possible in the sequences between the β strands?

Question 4.6
Given that the β strands in the β sheet structure of Problem 5 contain hydrophobic residues in most of the even-
Question 4.7
Ramachandran plots can help increase the accuracy of models derived from x-
130
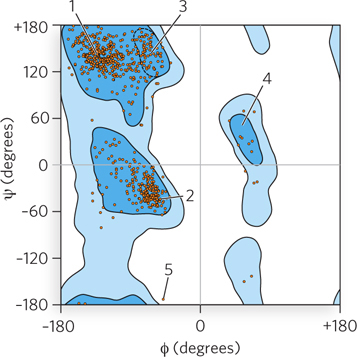
Question 4.8
Inspect the 20-
AIPRKKREFICRFGAIRPNT
Question 4.9
Predict which of the following sequences would bind ATP (or GTP), and explain your answer. (See the How We Know section in this chapter.)
YLFGGTRGVGKTSIA
LLIQALPGMGDDARL
LLIFGPPGLPKTTKL
FINAGSQGIGKTACL
Question 4.10
A polypeptide chain has 140 amino acid residues. How long will the polypeptide chain be if it is entirely α-helical? How long will it be if it is one continuous β strand?
Question 4.11
Compare and contrast four aspects of the use of NMR and x-
Question 4.12
Five proteins are listed below, each a monomer containing the number of amino acid residues indicated. How many domains would you expect each protein to have? Explain your reasoning.
70
110
150
200
250
Question 4.13
For the following 20-
DLKFTISVGAPVLTREQLLE
Question 4.14
Consider an α helix in isolation, apart from the rest of the protein:
NRGAAEGAFCRAN
How would the following amino acid substitutions (indicated by residue number) affect the stability of the helix?
Change N1 to K.
Change N1 to E.
Change R2 to K and E6 to R.
Change R2 to K and E6 to D
Change both G3 and G7 to F.
Change G7 to P.
Question 4.15
A simple protein structure is shown below, from two different angles. In (a), label the N-
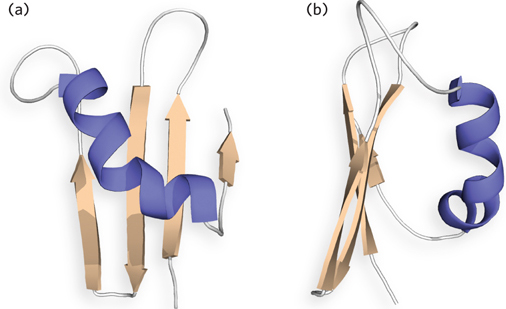
Question 4.16
Draw a topology diagram for a 10-
Question 4.17
Three Ramachandran plots are shown below. One of these is a guide; the other two, (a) and (b), are plots for two proteins, also shown below. Match each Ramachandran plot with its protein: which plot is most likely to be derived from bovine serum albumin, and which from green fluorescent protein? Both plots were downloaded from the Protein Data Bank. They are drawn a bit differently, but the axes are the same as in the guide.
131
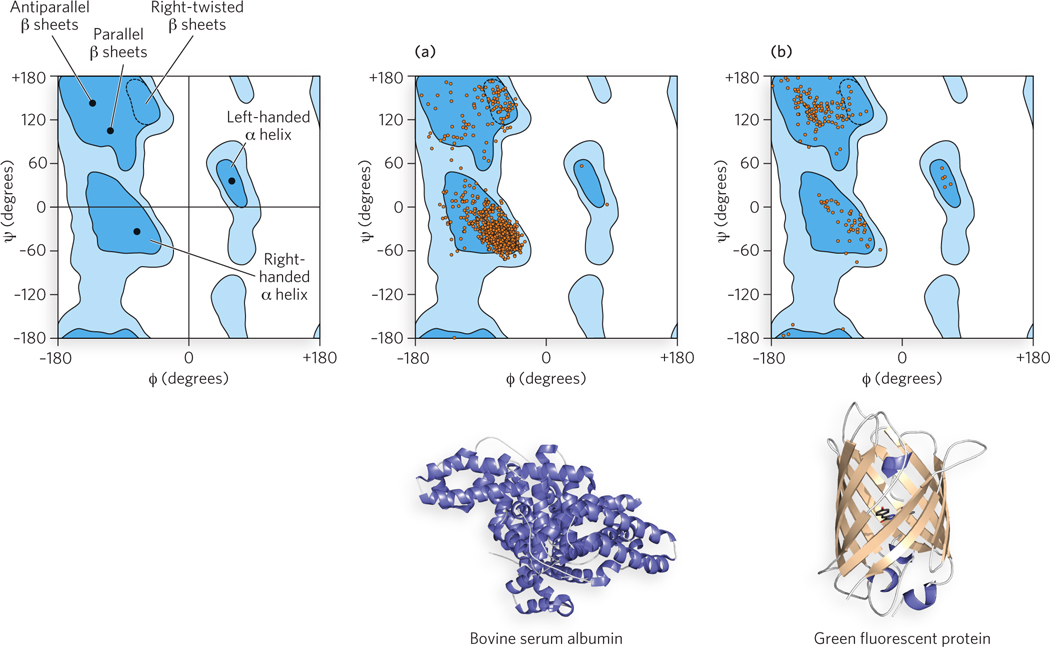
Question 4.18
When proteins are synthesized in cells, all of the peptide bonds are synthesized in the trans configuration. Some proteins, however, have peptide bonds—
Question 4.19
Food for thought: In this chapter, we introduce quaternary structure as the assembly of multiple protein subunits. This assembly is generally enforced through weak, noncovalent interactions—