Fruit Fly, Drosophila melanogaster
We are all familiar with the fruit fly as a cosmopolitan nuisance, but Drosophila melanogaster has a 100-year history as an important model organism for studying genetics and development. The fruit fly’s body is divided into several segments that form three major sections: the head, thorax, and abdomen. The body sections are encased in a hard chitin cuticle, secreted by underlying epidermal cells, and is rich in anatomic details (indentations, hairs) that serve as phenotypic landmarks for genetic studies.
Drosophila is small (about 2.5 mm long); it is easily and inexpensively grown in the laboratory, in bottles containing a layer of cornmeal, molasses, and yeast. The fruit fly has a reasonably rapid generation time (12 days), is simple to cross by mating, and produces hundreds of progeny in each generation. The main inconvenience for researchers is its ability to fly away. Although Drosophila was originally used to study the basic mechanisms of transmission genetics, many new genetic tools can now scrutinize the developmental basis of embryogenesis and the body plan.
Early Studies of Drosophila as a Model Organism
In 1908, Thomas Hunt Morgan looked for a suitable organism in which to study animal genetics. With little funding for science available at that time, he eventually settled on Drosophila because it was cheap and inexpensive to grow. In 1910, after two years of fruitless studies, Morgan found a male white-eyed spontaneous mutant (the flies normally have red eyes). Elegant and detailed studies of this single mutant revealed that genes are located on chromosomes, that each gene has two alleles, that alleles assort independently during meiosis, and that genes located on different chromosomes assort independently. These and other important findings supported Mendel’s laws in the physical context of genes located on chromosomes (see Chapter 2).
The fruit fly is also the first organism for which a genetic map was constructed. Alfred Sturtevant, a student in Morgan’s laboratory, mapped the relative distance between genes along chromosomes, based on their frequencies of crossover recombination. Calvin B. Bridges carried the studies further by identifying the exact positions of genes within polytene chromosomes. These giant chromosomes, located in the fruit fly’s salivary glands, consist of bundles of chromosomes packed together and have unique banding patterns when stained (Figure A-7). Bridges identified more than 5,000 bands arranged in a distinct pattern. We do not know why polytene chromosomes form, but it may be related to the job of the salivary gland in excreting the pupal casing for metamorphosis. Numerous recombination-based mutations were traced to missing bands or to spots where chromosomes had inverted, enabling the mapping of genes along a chromosome, as well as solidifying the location of genes on chromosomes.

Figure A-7: An insect polytene chromosome.
Flies have a short (12 day) diploid life cycle (Figure A-8). Sex in flies is determined by X chromosome copy number, not by the Y chromosome (XX is female, XY and the rare X0 are male), although the Y chromosome is required for the production of sperm. About a day after mating, the female begins to lay hundreds of eggs. Nuclear divisions in the embryo are the most rapid of any multicellular organism, and eggs hatch in about one day. The maggot proceeds through three larval instar stages, separated by molts, that take about 5 days. Larvae contain imaginal discs of tissue that are destined to become each of the appendages of the adult fly (e.g., eyes, antennae, legs, wings, halteres (modified wings acting as flight stabilizers), and mouthparts). The pupal case is derived from the larval cuticle, within which metamorphosis occurs in 3½ to 4½ days to yield the adult fly. Flies live approximately 30 days.
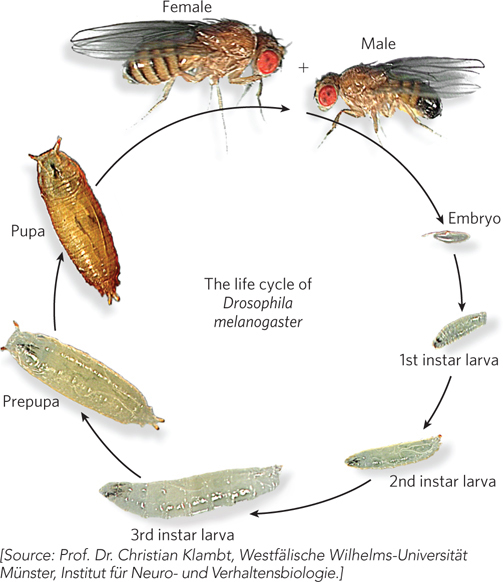
Figure A-8: The life cycle of Drosophila melanogaster.
Mutagenesis Flies can be mutagenized by exposure to ionizing radiation or by feeding with mutagenic chemicals. Mutations that result in changes in eye color, wing shape, or body parts can be identified by visual screening.
Introduction of DNA A process known as P-element transformation is used to insert recombinant DNA, and one application of this approach is to study the effects of protein expression on transcriptional control elements. The P element is a 3 kbp transposon that can carry a section of recombinant DNA between its terminal repeats in place of the self-encoded transposase and repressor (see Chapter 14). The recombinant P-element DNA is injected into the fertilized egg, along with a transposase-encoding plasmid. Insertion is random, and the plasmid encoding the transposase gene is lost during cell divisions, thereby preventing reinsertion of the transposon elsewhere in the genome.
Balancer Chromosomes Recessive lethal alleles can be stably maintained in fruit flies when paired with a balancer chromosome—a chromosome that cannot recombine with its homologous pair, due to the presence of many internal inversions that prevent complete alignment during meiosis. Balancer chromosomes are homozygous lethal, so the only progeny that survive are heterozygous for the recessive lethal gene and the balancer.
Genetic Mosaics Genetic mosaics, patches of genetically altered tissue, can be formed in adult or developing flies by x-ray irradiation to induce mitotic recombination. Mosaics are particularly useful in the study of lethal genes. Genetic mosaics of lethal genes can also be formed through the use of a heat-inducible yeast recombinase (called FLP) engineered into the genome. Heat induction results in a high frequency of mitotic recombination to form genetic mosaics. Although the genetic alterations may be lethal to embryos, they do not necessarily kill the adult, given the localized expression in only some cells.
Gene Knockouts and Gene Replacements Gene knockouts are obtained by homologous recombination, which occurs infrequently in Drosophila. The experimental strategies to edit genes in the fly genome depend on homologous recombination. Researchers inject into the fly embryo a modified copy of the targeted gene. A double-strand break in the gene is generated, and during its repair by homologous recombination, the wild-type gene is replaced by the modified copy. More recently, researchers have demonstrated that the CRISPR/Cas system can be used to directly edit endogenous genes, eliminating the need to go through this two-step approach.
RNA Interference RNAi can be used to effectively knock down specific gene products in lieu of a true gene knockout. RNAi can also be employed to knock down a gene product in a specific tissue at a specific time.
Drosophila as a Model Organism Today
Human Disease The ∼170 Mbp Drosophila genome is about one-twentieth the size of the mouse and human genomes, yet it encodes nearly the same number of gene families. About 60% of the genes known to be involved in human disease have homologs in the fruit fly. For example, studies of embryonic lethal genes in Drosophila have helped explain the genetic basis of human birth defects. Other disease models include immunological disorders, diabetes, cancer, Huntington disease, Alzheimer disease, and Parkinson disease.
Body Plan Development Localization of maternal mRNA in eggs results in localized gene expression that sets up the anterior-posterior and dorsal-ventral axes in Drosophila. These genes control the body plan and have counterparts in more complex animals. Thus, the fly serves as a relatively simple system to understand body plan formation (see Figure 16-24; see also Chapters 21 and 22).
Behavior Drosophila provides a model for understanding the cellular and molecular basis for certain types of behavior. Fruit fly behavioral abnormalities include changes in learning and memory, foraging, resting behavior, sleep, and alcohol consumption, among other behaviors.


