10.1 NUCLEOSOMES: THE BASIC UNITS OF DNA CONDENSATION
Scientists have been fascinated with the structure and behavior of chromosomes for more than 100 years. Chromosomes were first made strikingly visible through the use of dyes that stain specific subcellular structures. The deeply stained colored bodies (thus the name “chromosomes”) appeared in pairs and separated into two new daughter cells at cell division, correlating with Mendel’s observations that the particles of heredity come in pairs. As we learned in Chapter 2, scientists eventually came to understand that DNA is the hereditary substance inside chromosomes.
The material of chromosomes, both protein and DNA, is often referred to as chromatin. The protein component is about equal in mass to the DNA component. Some chromatin proteins are SMC proteins (see Chapter 9), topoisomerases, and transcription regulatory molecules; however, the largest protein component of chromatin is the histones. Histones are highly conserved, basic proteins that assemble into octameric complexes containing two each of four different histone subunits. DNA wraps around the histones to form condensed nucleosomes. Beginning with nucleosomes, eukaryotic chromosomal DNA is packaged into a succession of higher-order structures that ultimately yield the compact chromosome seen under the light microscope (see, for example, Figure 2-8, which shows the chromosomes of plant cells).
Histone Octamers Organize DNA into Repeating Units
Evidence that DNA is packaged into regularly organized units came from several types of studies. In one approach, chromosomal DNA was treated with a nonspecific DNA nuclease, such as micrococcal nuclease, that cuts DNA wherever it is not associated with proteins. The digested DNA fragments were then analyzed for size in an agarose gel. If DNA were packaged by proteins into units of a particular size, the nuclease would cleave only the DNA between these units, and the protected DNA segments (i.e., those bound to protein) would migrate in the gel as a ladder of unit-sized bands. If there were no regular repeating unit of protein-DNA packaging and proteins were distributed on DNA in a random way, then nuclease digestion would produce a smear of DNA fragments with no regular pattern. The results of the experiments revealed a series of regularly spaced DNA bands about 200 bp apart, indicating that DNA is packaged by proteins into units that encompass approximately 200 bp (Figure 10-1).
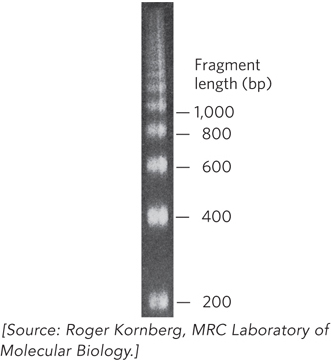
Figure 10-1: Evidence of DNA packaging into repeating units obtained from an experiment using a nuclease. Carefully isolated chromatin was treated with micrococcal nuclease and analyzed by agarose gel electrophoresis. The result was a DNA ladder of fragments that differed in length by 200 bp, suggesting that DNA packaging involves a repeat unit of 200 bp.
When the protein-DNA units, or nucleosomes, were examined by SDS-polyacrylamide gel electrophoresis (SDS-PAGE), four histone proteins (designated H2A, H2B, H3, and H4) were found in approximately equimolar ratios (Figure 10-2). A fifth histone (H1) was present in about half the amount relative to the other four histones. The five histones have molecular weights (Mr) between 11,000 and 21,000 (Da). Histones are rich in the basic amino acids arginine and lysine, which together make up about 25% of the amino acid residues in any given histone protein (Table 10-1). The amino acid sequences of histone proteins are highly conserved among eukaryotic organisms. Histones H3 and H4 are nearly identical in all eukaryotes, suggesting strict conservation of their functions. For example, only 2 of the 102 amino acid residues differ between the H4 histones of peas and cows, and only 8 residues differ between the H4 histones of humans and yeast. Histones H1, H2A, and H2B show less sequence similarity among organisms, but on the whole, they are more conserved than other types of proteins. Eukaryotes also have several variant forms of certain histones, notably histones H2A and H3, which, as we see later in the chapter, have specialized roles in DNA metabolism.
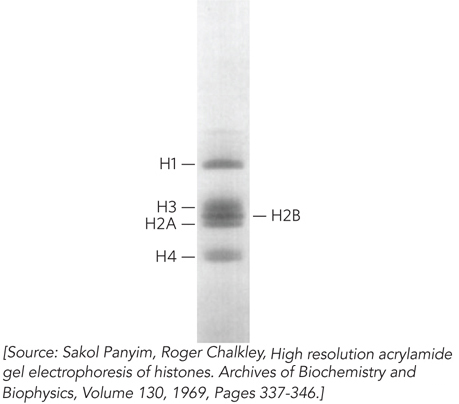
Figure 10-2: Histone representation in nucleosomes. Histones were extracted from chromatin, and the histone subunits were separated by SDS–polyacrylamide gel electrophoresis. Measurement of the band intensity showed histones H2A, H2B, H3, and H4 present in equal amounts, and histone H1 at about half the level of the other histones.
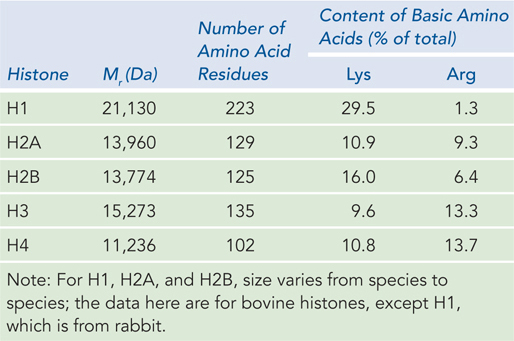
Figure 10-1: Types and Properties of Histones
To understand how the histones are organized within the nucleosome, the native state of the nucleosome unit must be preserved. Early studies of nucleosomes used denaturing methods of extraction that disrupted their native state. Later, by extracting chromatin with mild salt solutions (2 m sodium chloride and 50 mm sodium acetate), researchers kept nucleosomes intact and could investigate the composition and organization of the nucleosome unit.
Some of the key studies involved protein cross-linking. In this technique, a chemical with two reactive groups is used to react with a protein complex. Because the chemical has two reactive groups, it can covalently attach to two proteins, but because the chemical is a small molecule, it can only react with two proteins that are close together. Thus, identification of the cross-linked proteins reveals which proteins are next to each other in an oligomer. Based on findings from these procedures, Roger Kornberg proposed how histones are organized within the nucleosome (see the How We Know section at the end of this chapter).
Kornberg suggested that most of the 200 bp DNA segment in a protein-DNA unit is wrapped around a histone octamer composed of two copies each of histones H2A, H2B, H3, and H4. These four histones have come to be known as the core histones (Figure 10-3a). The remainder of the DNA serves as a linker between nucleosomes, to which histone H1 binds. Early studies of nucleosome-DNA structure included visualization of nucleosomes in the electron microscope by Ada Olins and Donald Olins at Oak Ridge National Laboratory. These studies revealed a structure in which the DNA is bound tightly to beads of protein, often regularly spaced like beads on a string (Figure 10-3b)—a result that defined the nucleosome as a basic unit of DNA compaction.
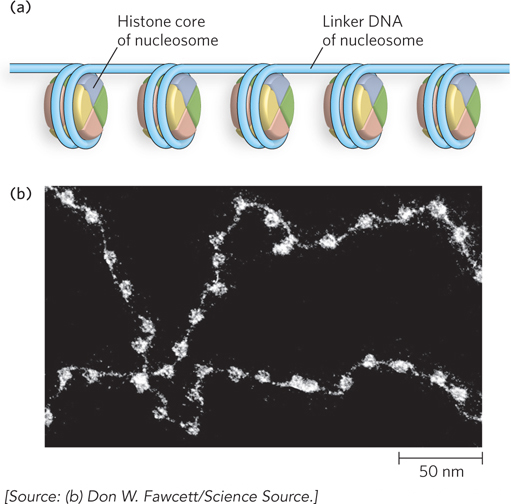
Figure 10-3: Nucleosomes as beads on a string. (a) Regularly spaced nucleosomes consist of core histone proteins bound to DNA. (b) In this electron micrograph, the DNA-wrapped histone octamers are clearly visible, with linker DNA between them.
Under physiological conditions, formation of the histone octamer from individual histone proteins requires the presence of DNA. In the absence of DNA, the highly conserved H3 and H4 subunits form a tightly associated heterotetramer that contains two of each subunit, and the less conserved H2A and H2B subunits form a heterodimer. Without DNA, these components do not assemble into a histone octamer unless incubated under nonphysiological conditions at high salt concentrations. In the presence of DNA, however, two H2A-H2B heterodimers assemble with one H3-H4 heterotetramer and the DNA to form the nucleosome.
DNA Wraps around a Single Histone Octamer
The crystal structure of a histone octamer in a complex with 146 bp of DNA reveals the detailed architecture of a nucleosome particle (Figure 10-4). The most striking feature is the tight wrapping of DNA around the octamer in the form of a left-handed solenoidal supercoil (described in Chapter 9). Overall, the supercoil arrangement of DNA on the nucleosome compacts the DNA six- to sevenfold. The DNA is not uniformly bent, but instead follows a pattern of relatively straight 10 bp segments joined by bends.
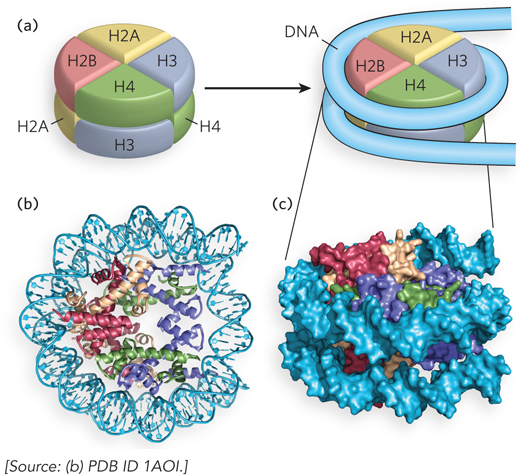
Figure 10-4: Structural model of the nucleosome. (a) The simplified structure of a nucleosome octamer (left), with DNA wrapped around the histone core (right). (b) A ribbon representation of the structure of the nucleosome from the African frog Xenopus laevis, with different colors representing the five histones, matching the colors in (a). The view in (b) is rotated relative to the view in (a) to show one face of the nucleosome. (c) Surface representation of the nucleosome. A 146 bp segment of DNA binds in a left-handed solenoidal supercoil that circumnavigates the histone complex 1.67 times. The orientation in (c) is the same as that in (a) to show the DNA wrapping around the nucleosome.
Each histone contains a histone-fold motif, three α helices linked by two short loops (Figure 10-5). The elemental structural unit of the nucleosome is a head-to-tail dimer of histone-fold motifs of either the H3-H4 pair or the H2A-H2B pair. Each histone-fold dimer forms a V-shaped structure that contains three DNA-binding sites (Figure 10-6a). The octamer structure shows that the connections between each dimer (the two H3-H4 pairs and two H2A-H2B pairs) of the four core histones are also mediated mainly by the highly conserved histone fold (Figure 10-6b).
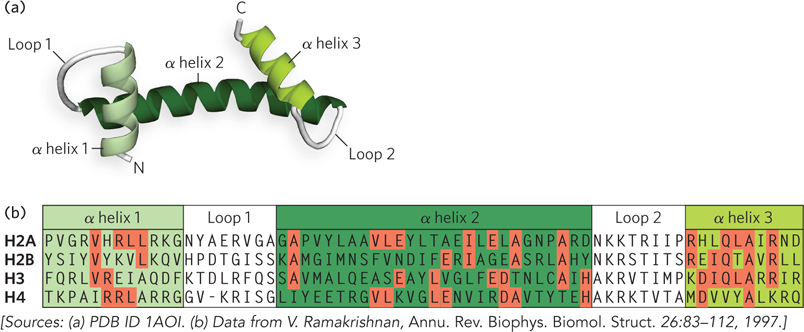
Figure 10-5: The histone-fold motif. (a) This internal structure of each core histone is formed from three α helices connected by two loops. (b) The amino acid sequences of the histone folds of the four core histones. Residues that are identical in the different histone subunits are shaded in red.
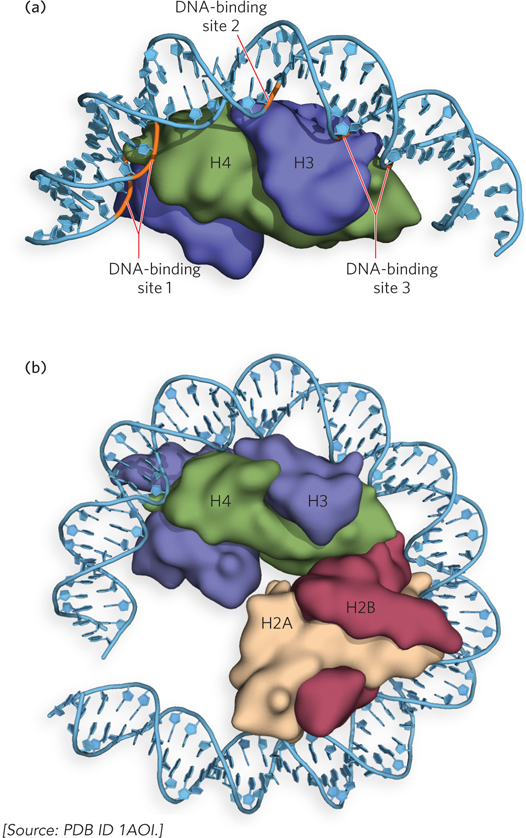
Figure 10-6: The histone-fold dimer. (a) A ribbon representation of the histone folds of one pair of H3-H4 subunits within the octamer, bound to DNA. DNA is in contact with the three DNA-binding sites formed by the two histone folds (i.e., the histone-fold dimer). (b) One face (one half) of the nucleosome contains two histone-fold dimers.
The contacts between histones and DNA are mainly between the conserved histone fold and the phosphodiester backbone or minor groove of the DNA, in keeping with the relatively nonspecific binding of nucleosomes to DNA. Approximately half of the 142 hydrogen bonds of the histone fold occur between the DNA and peptide backbone atoms, rather than amino acid side chains. This seems counterintuitive, given the many basic side chains of histones. A possible explanation is that amino acid side chains are not as rigidly held in place as is the peptide backbone, and therefore hydrogen bonding of DNA to peptide backbone atoms more firmly secures the DNA to the protein. The basic histone side chains are needed to neutralize the negative charge of the DNA’s phosphodiester backbone. Charge neutralization is important in DNA condensation, especially in the further stages of compaction.
The average DNA twist when wrapped around the histone octamer is 10.2 bp per turn, compared with the 10.5 bp per turn of unrestricted DNA; thus, the DNA structure must adapt to the octamer. This overtwisting of DNA results in a narrowing of the minor groove. The change in shape of the DNA as it binds the histone octamer through bending and overtwisting implies that the octamer is more likely to bind DNA sequences that readily conform to such changes. For example, a local abundance of A=T base pairs in the minor groove of a DNA helix, where it is in contact with the histones, facilitates the compression of the minor groove that is needed for tight wrapping of DNA around the histone octamer (Figure 10-7). In fact, histone octamers assemble particularly well with sequences where two or more A=T base pairs are staggered at 10 bp intervals, because DNA is naturally bent at these sequences, and where two or more consecutive A=T base pairs are spaced along the same face of the helix, the DNA bends into a circle. Tracts of G≡C base pairs have the opposite effect, preventing compression of the minor groove, and thus are preferred at positions not facing nucleosomes.
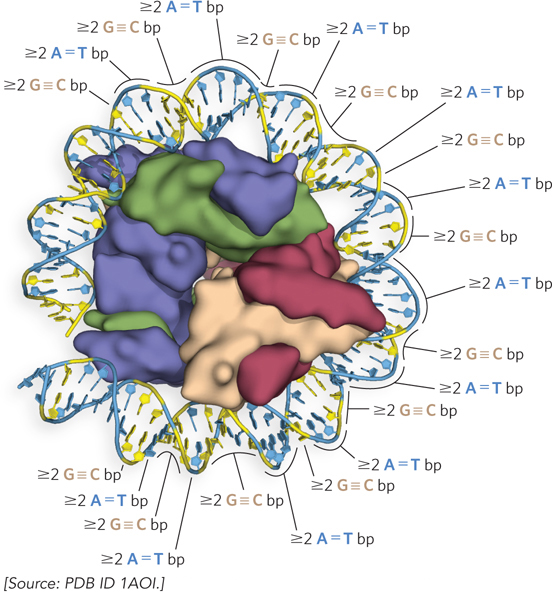
Figure 10-7: The effect of DNA sequence on nucleosome binding. Runs of two or more adjacent A=T base pairs facilitate the bending of DNA, whereas runs of two or more G≡C base pairs have the opposite effect. When spaced at about 10 bp intervals, consecutive A=T base pairs help bend DNA into a circle. When consecutive G≡C base pairs are spaced 10 bp apart and offset by 5 bp from runs of A=T base pairs, the DNA binds tightly to the nucleosome.
The left-handed solenoidal supercoil of the 146 bp duplex that winds 1.67 times around the nucleosome reveals why eukaryotic DNA is underwound, even though eukaryotic cells lack topoisomerases that underwind DNA. Recall that the solenoidal wrapping of DNA is just one form of supercoiling that underwound (negatively supercoiled) DNA can assume (see Chapter 9). The tight wrapping of DNA around the histone core requires the removal of about one helical turn in the DNA. When the histone core of a nucleosome binds in vitro to relaxed, closed-circular DNA, the binding introduces a negative supercoil. Because binding in this fashion does not break DNA or change the linking number, formation of the negative supercoil around the histones must be accompanied by a compensatory positive supercoil in the unbound region of the DNA (see Figure 9-22). As described in Chapter 9, eukaryotic topoisomerases can relax positive supercoils and are required for the assembly of chromatin from purified histones and closed-circular DNA in vitro. Relaxing the unbound positive supercoil leaves the negative supercoil fixed through its binding to the nucleosome histone core. Overall, this results in a decrease in linking number.
Histone Tails Mediate Internucleosome Connections That Regulate the Accessibility of DNA
Most of the mass in the histone octamer forms a tightly packed particle, but the N-termini of the histones protrude from the core particle and are less ordered (Figure 10-8). These N-terminal histone tails are flexible and therefore mostly disordered in the crystal structure. The parts of the histone tails that are visible in the crystal structure appear as irregular chains extending outward from the nucleosome disk. The tails exit the DNA superhelix through channels formed by the alignment of minor grooves of adjacent DNA helices every 20 bp. The histone tails do not contribute much strength to DNA binding, but they form intermolecular contacts with adjacent nucleosome particles and organize nucleosomes into a higher-order chromatin structure (Figure 10-9).
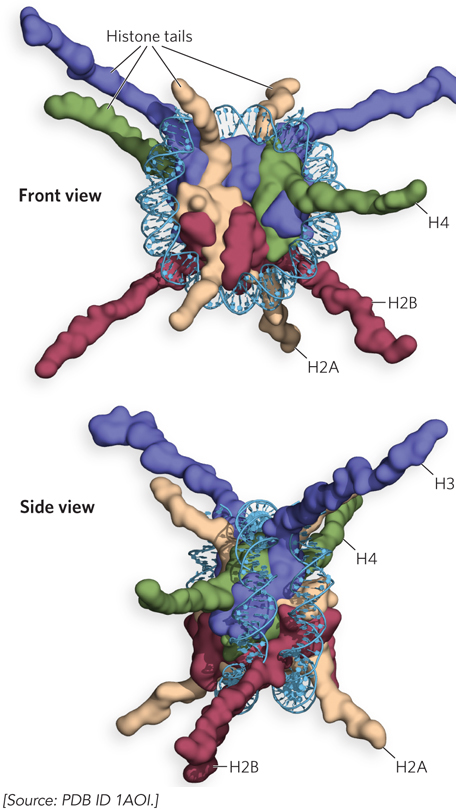
Figure 10-8: Histone tails. Two views of histone N-terminal tails protruding from between the two DNA duplexes that supercoil around the nucleosome. (a) Ribbon representation, looking at the circular face of the nucleosome. (b) Space-filling representation of the nucleosome as viewed from the side. Some tails pass between the supercoils through holes formed by alignment of the minor grooves of adjacent helices. The H3 and H2B tails emerge between the DNA turns wrapped around the histone; the H4 and H2A tails emerge between adjacent histone cores.
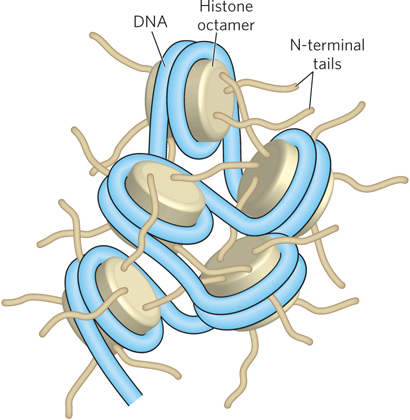
Figure 10-9: Internucleosomal contacts through N-terminal tails. The N-terminal tails of the histones of one nucleosome protrude from the particle and interact with adjacent nucleosomes, resulting in higher-order DNA packaging.
Chromatin structure is far from static and can, in fact, change in quite a dynamic fashion with changes in the environment and state of cellular differentiation. The ability of chromatin to change structure affects gene expression and is a very active area of research. The histone tails are at the heart of the dynamic regulation of chromatin structure, because they are the target of numerous chemical modifications that control the access of regulatory proteins to DNA. These modifications affect the net electrical charge, shape, and other properties of histones, which in turn affect the structural and functional properties of chromatin. Histone tails can also be recognized by particular enzymes. As we will see, these modifications play important roles in the regulation of transcription, replication, and DNA repair.
Histone tail modifications impact the molecular interactions between adjacent nucleosomes, thereby changing the level of chromatin compaction and thus the access of proteins to the altered chromatin structure. The tighter the internucleosome connections mediated by the histone tails, the less accessible DNA is to transcription factors and other proteins. Histone H1 also plays a role in DNA sequestration. Histone H1 is not as extensively modified by chemical groups as the core histones are, but it facilitates the general repressive effect of histones on transcription. This effect can be demonstrated experimentally in an in vitro assay that uses a cellular transcription extract, where transcription can proceed when DNA templates are added. As shown in Figure 10-10, plasmid DNA containing the kruppel gene of Drosophila melanogaster was transcribed in the absence (lanes 1 and 2) or presence (lanes 3 to 10) of histones. Adding the core histone proteins resulted in decreased RNA synthesis (compare lanes 1 and 3). Adding the linker histone H1 also had a profound effect: as histone H1 was titrated into the assay (as shown along the top of Figure 10-10), transcription was diminished even further (compare lanes 1, 3, and 5).
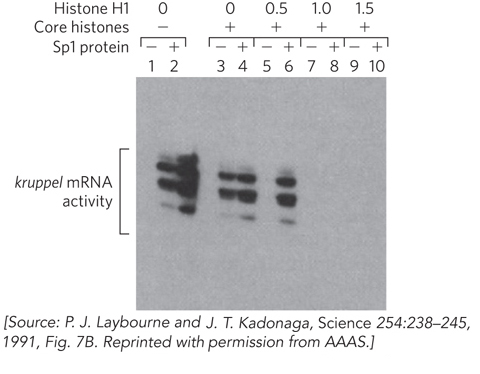
Figure 10-10: The repression of transcription by histones. An in vitro transcription extract is used to monitor transcription from a promoter in the presence or absence of histone proteins and the transcription activator Sp1. The first two lanes are controls in the absence of histones: transcription is optimal, and Sp1 stimulates. Lanes 3 and 4 are also controls, showing results with core histones present but no H1. In lanes 5 to 10, histone H1 was added in concentrations of 0.5, 1.0, or 1.5 H1 molecules per nucleosome. Sp1 prevents gene repression by binding specific sequences in the promoter.
Transcription factors that bind specific sites on DNA can modulate the repressive effect of histones on transcription. The site-specific DNA-binding protein Sp1, which enhances transcription, protects against histone-mediated transcription repression (compare lanes 5 and 6 in Figure 10-10). One may infer that Sp1 binds DNA and blocks the binding of histones to DNA in its immediate vicinity. However, Sp1 is not effective in the presence of higher levels of H1 (compare lanes 6, 8, and 10).
SECTION 10.1 SUMMARY
Chromatin refers to the protein and DNA components that comprise the material of chromosomes.
The basic unit of DNA packaging is the nucleosome, composed of DNA wrapped around a histone octamer.
The histone octamer contains a tetramer of histones H3-H4 and two dimers of histones H2A-H2B. The amino acid sequences of the histones are highly conserved among eukaryotes.
Nucleosomes are linked together by DNA with one bound molecule of H1. The spacing of nucleosomes along DNA results in a beads-on-a-string appearance as seen by electron microscopy.
DNA is wrapped around the histone octamer in a left-handed solenoidal supercoil, making 1.67 turns. Histones contain a conserved histone-fold motif, which contacts the DNA (mainly the phosphodiester backbone and minor groove).
N-terminal histone tails are flexible and can be modified to affect DNA transcription. These modifications probably alter the molecular interactions of adjacent nucleosomes, thereby changing the access of proteins to DNA in the altered chromatin structure.










