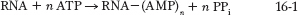16.1 MESSENGER RNA CAPPING AND POLYADENYLATION
In bacteria, mRNAs are often translated into protein as they are being transcribed, typically without any posttranscriptional modification. In eukaryotes, however, transcription occurs in the nucleus, and mRNAs must be transported to the cytoplasm before translation can occur. This uncoupling of transcription and translation provides opportunities for the cell to regulate when and where mRNAs are translated, and also requires that cells guard against premature degradation of the mRNA. One important way that eukaryotic cells achieve both regulation and protection of mRNAs is by chemically capping the 5′ end and adding a string of adenosines to the 3′ end. In addition to preventing degradation of the mRNA, these modifications provide chemical handles for subsequent steps in pre-mRNA (precursor mRNA) processing events and nuclear export.
Eukaryotic mRNAs Are Capped at the 5′ End
RNA polymerase II (Pol II) synthesizes single-stranded ribonucleic acids that are vulnerable to degradation at either end by exoribonucleases. The first modification made to a pre-mRNA transcript is the addition of a 5′ cap, which prevents degradation from the 5′ end. The 5′ cap is a residue of 7-methylguanosine (7-meG) linked to the 5′-terminal residue of the mRNA through an unusual 5′,5′-triphosphate linkage. The 5′ cap helps protect mRNA by preventing recognition of the 5′ end by exoribonucleases, which digest RNA sequentially from a free 5′ or 3′ end.
The 5′ cap is formed by condensation of a molecule of GTP with the triphosphate at the 5′ end of the transcript. The guanine is subsequently methylated at N-7, and additional methyl groups are often added at the 2′ hydroxyls of the first and second nucleotides adjacent to the cap (Figure 16-2a). The methyl groups are derived from S-adenosylmethionine. All of these reactions occur very early during transcription, after the first 20 to 30 nucleotides of the transcript have been added (Figure 16-2b). Four different variants of the capping enzyme, guanylyltransferase, carry out the capping reaction. Only RNAs made by RNA Pol II are capped, because all four guanylyltransferase enzymes are associated with the CTD of Pol II (not with RNA polymerase I or III).
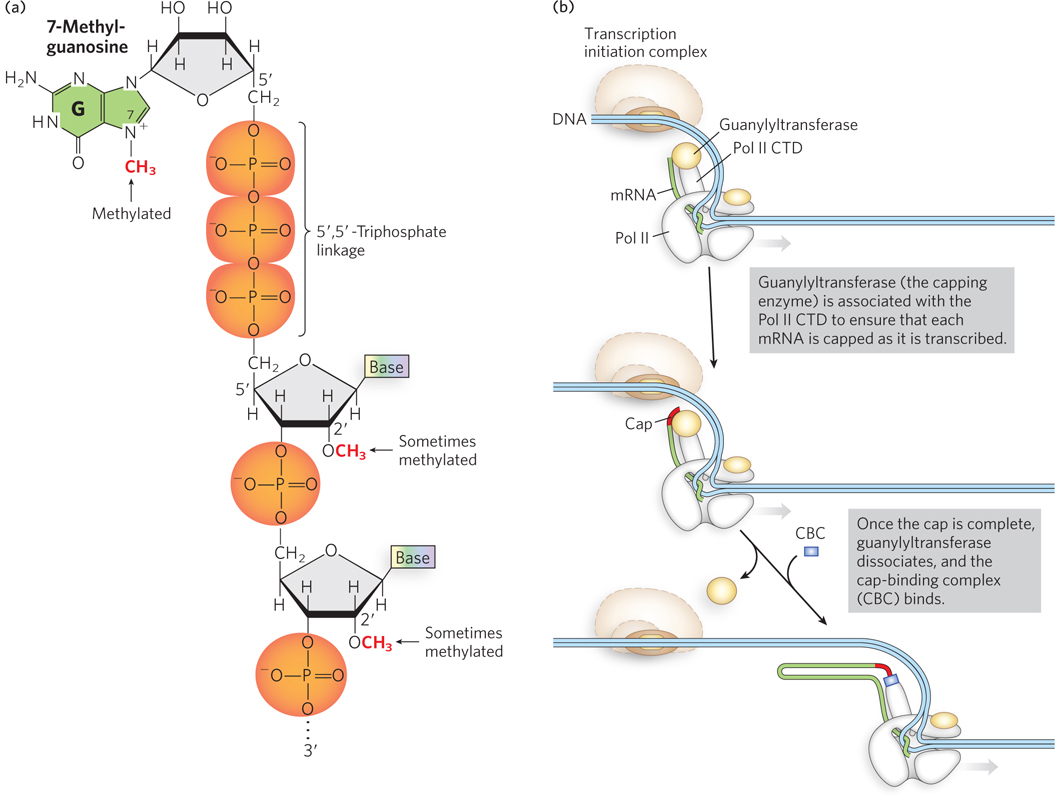
Figure 16-2: The mRNA 5′ cap. (a) The chemical structure of the 5′ cap of mRNA, showing the 7-methylguanosine and adjacent residues. (b) The cap is synthesized by the capping enzyme, guanylyltransferase, which is associated with the C-terminal domain (CTD) of RNA polymerase II.
In the 1970s, experiments by Aaron Shatkin and his colleagues showed that the 5′ cap also plays a critical role in binding the mRNA to a ribosome. Using mRNAs isolated from reovirus, a human virus that can cause stomach flu, Shatkin’s research group found that only mRNAs containing a 5′ cap are competent to initiate protein synthesis. Viral mRNAs lacking a 5′-methylated terminus are never part of actively translating ribosomes, because they fail to bind to the 40S ribosomal subunits during the translation initiation process (we discuss the details of protein synthesis in Chapter 18). Shatkin and colleagues used the reovirus RNA polymerase to synthesize reoviral mRNAs, using [32P]GTP and [3H]methyl-S-adenosylmethionine. Some of the resulting transcripts contained a 5′-7-meG cap (and thus were 3H- and 32P-labeled) and the rest lacked a cap (32P-labeled only). These mRNAs were incubated with wheat germ cell extract to allow the mRNAs to bind to ribosomes. The samples were then deposited at the top of a sucrose density gradient and spun in a centrifuge, so that the samples migrated into the gradient according to their density (Figure 16-3). When the resulting gradients were fractionated, and each fraction measured for its content of 3H- and 32P-containing material, the capped mRNAs were found in the dense part of the gradient where ribosomes were located, indicating that the capped mRNAs were bound to the ribosomes. In contrast, the uncapped mRNAs (containing only 32P) migrated only to the top of the gradient. As we discuss in Chapter 18, the 5′ cap binds the cap-binding complex (CBC) (see Figure 16-2b), a specific complex of proteins that recruits capped mRNAs to the ribosome to initiate translation.
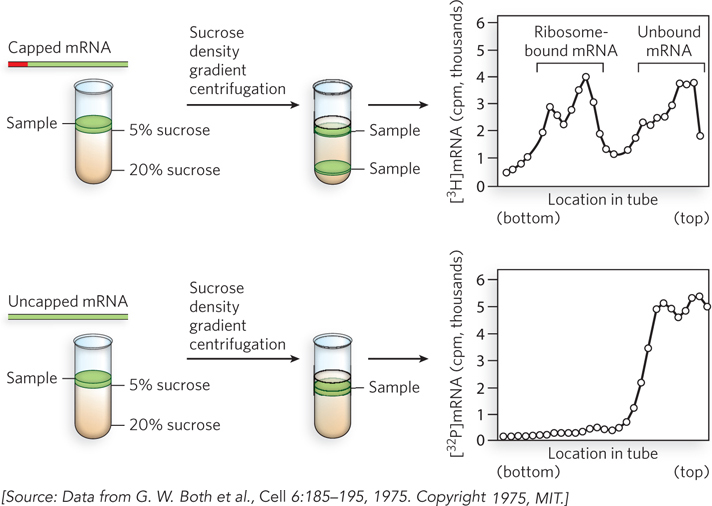
Figure 16-3: The 5′ cap requirement for ribosome binding and translation. Radiolabeled mRNAs with or without a 5′-methyl cap were incubated with ribosomes, and the complexes were separated from free mRNA by sucrose density gradient centrifugation. Denser particles migrate farther in the gradient, as shown on the left side of the graphs. Radioactivity levels (in counts per minute) of capped (top panel) and uncapped (bottom panel) mRNAs show that only the mRNA with a 5′ cap migrates with ribosomes.
Eukaryotic mRNAs Have a Distinctive 3′-End Structure
Like the 5′ end, the 3′ end of mRNAs must be protected from nucleolytic degradation. As we discussed in Chapter 15, mRNAs contain a polyadenylation signal embedded in the sequence transcribed by RNA polymerase. An enzyme associated with the C-terminal domain of Pol II cleaves the transcript after the polyadenylation signal, and the mRNA is polyadenylated by other CTD-associated factors. The rest of the transcript dissociates from the polymerase and is degraded.
The 3′ poly(A) tail, typically 80 to 250 A residues, serves as a binding site for one or more specific proteins that help protect mRNA from enzymatic destruction, by physically blocking the access of ribonucleases to the 3′ end. One way this was first demonstrated was by injecting mRNAs with or without a 3′ poly(A) tail into frog eggs and testing how long the mRNAs remained intact. The 3′-polyadenylated mRNAs survived for much longer, with a remarkable 40% remaining intact 48 hours after injection. Other experiments with mRNAs injected into egg cells or incubated in cell extracts showed that, like the 5′ cap, the poly(A) tail and its protein binding partners interact with the cap-binding complex during mRNA recruitment to the ribosome.
The poly(A) tail is added in multiple catalytic steps involving polyadenylation factors, polyadenylate polymerase (PAP) and poly(A) binding protein (PABP) (Figure 16-4). First, Pol II extends the transcript beyond the site where the poly(A) tail is to be added. The transcript is cleaved at the poly(A) addition site by an endonuclease component of a large enzyme complex, again associated with the CTD of Pol II. The mRNA site where cleavage occurs is marked by two sequence elements: the highly conserved sequence 5′-AAUAAA located 10 to 30 nucleotides on the 5′ side (upstream) of the cleavage site, and a less well-defined sequence rich in G and U residues located 20 to 40 nucleotides downstream from the cleavage site. Cleavage generates the free 3′-hydroxyl group that defines the end of the mRNA, to which A residues are immediately added in the PAP-catalyzed reaction:
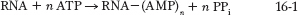
where n = 80 to 250. The number of A residues in the poly(A) tail varies from one species to another; it is often written simply as (A)n. The polyadenylation enzyme complex does not require a template, but it does require cleavage of the mRNA. A few types of eukaryotic mRNA, such as those that encode histones, are cleaved but have a different kind of 3′ tail that allows them to be regulated separately from the bulk of the cellular mRNA (Highlight 16-1).
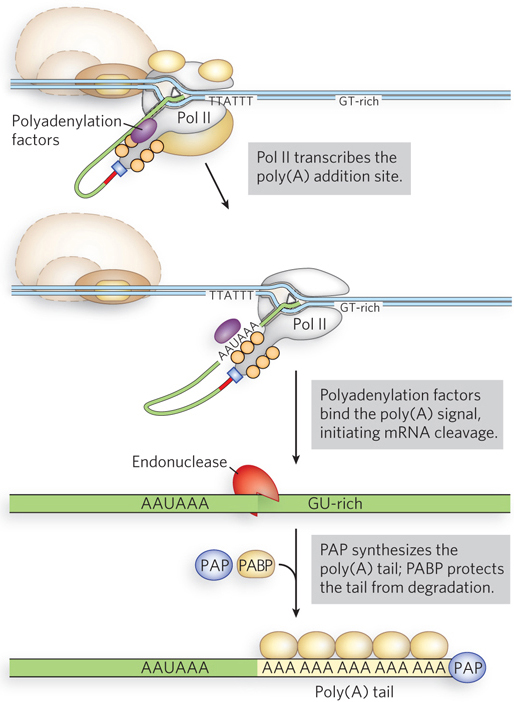
Figure 16-4: Addition of the 3′ poly(A) tail to the transcript. Polyadenylation factors are associated with Pol II during transcription. After transcription of the 5′-AAUAAA sequence, these proteins transfer to the transcript and help recruit cleavage factors and poly(A) binding protein (PABP) before dissociating. The poly(A) tail is synthesized by polyadenylate polymerase (PAP).
HIGHLIGHT 16-1 EVOLUTION: Eukaryotic mRNAs with Unusual 3′ Tails
Unlike most eukaryotic mRNAs, those that encode histones—the small basic proteins that help package eukaryotic chromosomal DNA (see Chapter 10)—lack 3′ poly(A) tails. Instead, the 3′ ends contain a self-complementary sequence that forms a stem-loop structure, creating the binding site for a protein complex that includes the stem-loop binding protein (SLBP) (Figure 1). SLBP both stabilizes the 3′ end of histone mRNAs, preventing degradation, and provides an alternative way of recruiting the mRNA to ribosomes during translation initiation. Nascent histone-encoding transcripts have 3′ extensions that are cleaved at a site five nucleotides after the conserved stem-loop, a process catalyzed by a specific endonuclease that cleaves internal bonds in the phosphodiester backbone. The upstream cleavage product corresponds to the mature histone mRNA, and the downstream product is degraded by an exonuclease, which cleaves RNA from the 5′ or 3′ end.
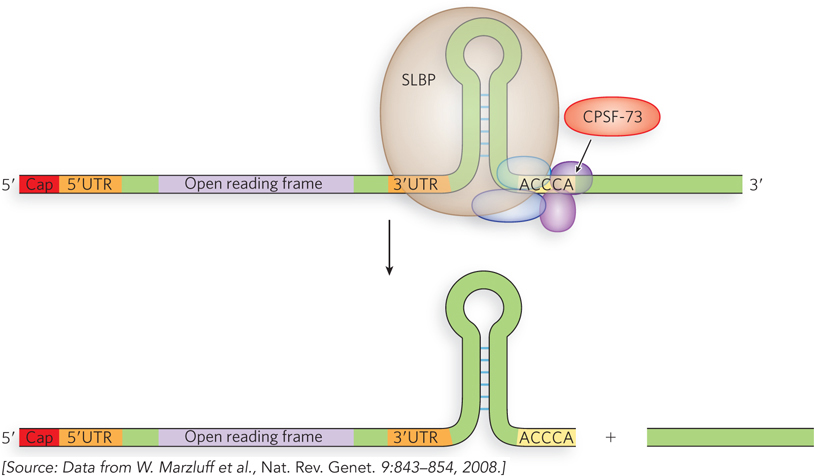
FIGURE 1 The 3′ stem-loop structure of a histone pre-mRNA binds the protein SLBP, which then assembles with CPSF-73 and other splicing factors to process the 3′ end. The open reading frame is the coding part of the gene.

William Marzluff
The processing of histone pre-mRNAs is interesting, because it provides a counterpoint to the more common pre-mRNA processing pathway and therefore may illuminate evolutionary connections between the two. To identify the ribonucleases responsible for these histone pre-mRNA processing events, William Marzluff and his colleagues at the University of North Carolina conducted a cross-linking experiment (Figure 2). Histone pre-mRNA was synthesized in the laboratory to include a radioactive 32P label and incubated in the presence of a total protein extract from human cells. The preparation was then exposed to ultraviolet light to induce formation of chemical bonds between the mRNA and any proteins that might have bound to it. Remarkably, the researchers found the so-called cleavage and polyadenylation specificity factor (CPSF-73), a known protein component of the machinery responsible for cleavage and polyadenylation of the vast majority of eukaryotic mRNAs. These studies suggested that CPSF-73 is both the endonuclease and the 5′→3′ exonuclease involved in histone pre-mRNA processing. The crystal structure of human CPSF-73, along with biochemical experiments on the purified protein, showed that CPSF-73 is also the enzyme responsible for cleaving the 3′ ends of all pre-mRNAs prior to polyadenylation. These findings revealed an unexpected biochemical connection between 3′-end formation in histone mRNAs and in other, polyadenylated mRNAs, implying a common ancestral mechanism for processing the 3′ ends of all animal mRNA transcripts.
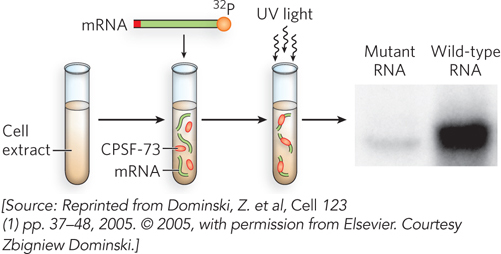
FIGURE 2 Cross-linking experiment to determine the protein bound to the radiolabeled stem-loop of histone mRNA. The cross-linked species were separated by denaturing gel electrophoresis, based on their slower migration relative to non-cross-linked molecules, revealing a prominent species that was found to contain CPSF-73. The cross-linked species corresponding to CPSF-73– pre-mRNA is present in the gel with the wild-type RNA, but is greatly reduced in a reaction that used a mutant RNA defective for 3′-end processing.
mRNA Capping, Polyadenylation, and Splicing Are Coordinately Regulated during Transcription
The regulation of mRNA capping, polyadenylation, and splicing is coordinated through the association of these processes with RNA polymerase II. Capping, splicing, and polyadenylation factors are associated with the Pol II C-terminal domain, and these factors transfer to the nascent RNA at the appropriate sequences. This enables RNA processing to occur simultaneously with transcription, ensuring that mature mRNAs are processed rapidly as they emerge from the Pol II active site (Figure 16-5). Once the 5′ end of the transcript has been capped, it is released from the capping enzymes and bound by the cap-binding complex, which itself associates with the Pol II CTD.
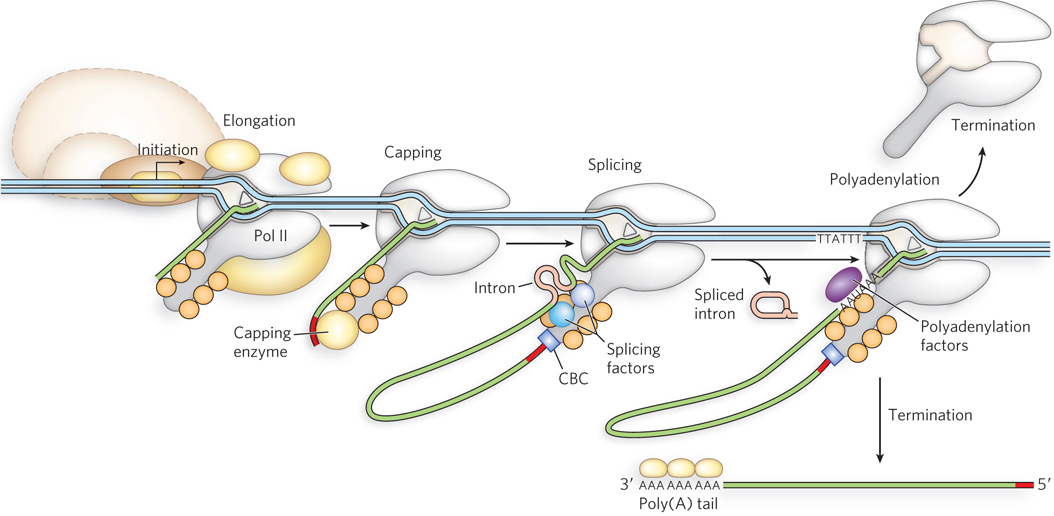
Figure 16-5: Coordination of transcription and pre-mRNA processing. mRNA-processing proteins associate with the C-terminal domain of Pol II. As the mRNA is synthesized, the capping enzyme (guanylyltransferase), splicing factors, and polyadenylation factors transfer from the Pol II CTD to the mRNA and process the mRNA as it is transcribed. (CBC is the cap-binding complex.) The mature transcript is used for multiple rounds of protein synthesis, then degraded.
In addition, some components of the splicing apparatus appear to be tethered to the Pol II CTD, suggesting an interesting model for the splicing reaction. As the first splice junction is synthesized, it is bound by a protein splicing factor. The second splice junction is then captured by this complex as it passes by, facilitating juxtaposition of the intron ends and the subsequent splicing process. After splicing, the intron remains in the nucleus and is eventually degraded (see Figure 16-5).
SECTION 16.1 SUMMARY
Eukaryotic mRNAs are modified by the addition of a 7-methylguanosine residue at the 5′ end and by cleavage and polyadenylation at the 3′ end to form a poly(A) tail.
Modification of the ends protects the transcript from ribonucleases and is required for subsequent steps in pre-mRNA processing, as well as for export from the nucleus.
Messenger RNA capping, polyadenylation, and splicing are coupled to transcription through the physical association of the enzymes with RNA polymerase II.