16.3 RNA TRANSPORT AND DEGRADATION
Once eukaryotic RNAs have been processed in the nucleus, they are ready for export to the cytoplasm. Mature mRNAs are translated into protein through the action of ribosomes, whereas other, non-protein-coding RNAs participate in various regulatory activities in the cytoplasm or undergo further modification in the cytoplasm then reenter the nucleus. In each case, the processed modifications of the nuclear RNAs identify them as being ready for export, and they are then transported to the cytoplasm.
Like other kinds of RNA processing, degradation is a highly regulated process carried out by complex enzymatic machinery. RNA degradation enzymes are essential to cell survival because they help maintain appropriate amounts of mRNAs in response to metabolic and environmental signals. These enzymes also rid the cell of defective mRNAs containing premature stop codons.
Different Kinds of RNA Use Different Nuclear Export Pathways
Nuclear export of most non-protein-coding RNAs involves members of a conserved family of transport receptors called importins and exportins, collectively known as karyopherins. Exportins bind to their RNA “cargo” in the nucleus and escort it through nuclear pores to the cytoplasm, where the cargo is released. Both tRNAs and some kinds of noncoding RNAs bind directly to their respective exportin. In contrast, rRNAs are exported in pre-ribosomal particles containing ribosomal proteins, several rRNA species, and nonribosomal proteins, using their own exportin. The snRNAs are transported to the cytoplasm and remain there only transiently, undergoing assembly into snRNP particles and then reentering the nucleus.
In each case, the exportin requires a small GTP-hydrolyzing protein called Ran that regulates cargo-receptor interactions (Figure 16-22). For nuclear export, the RNA cargo and exportin associate cooperatively with a Ran molecule bound to GTP. Once this ternary complex translocates to the cytoplasm, the Ran-bound GTP is hydrolyzed to GDP, causing release of the cargo RNA from the exportin. The Ran-GDP reenters the nucleus, where its GDP is exchanged for GTP by a guanine nucleotide exchange factor (GEF), and the cycle can begin again.
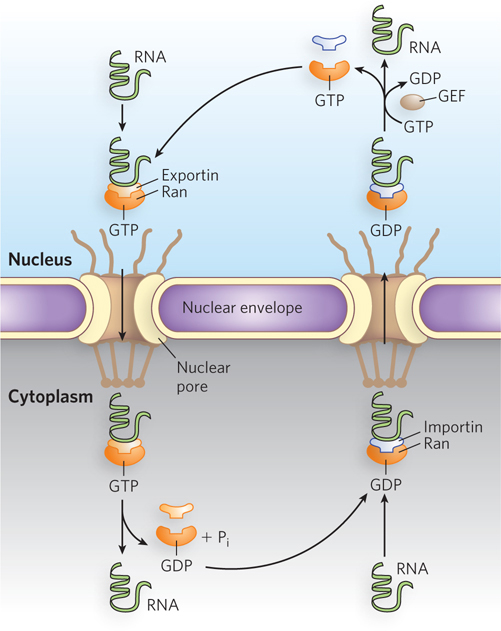
Figure 16-22: Nuclear export and import of RNA. The GTP-hydrolyzing protein Ran regulates RNA-receptor interactions. For nuclear export, the RNA cargo and its exportin receptor associate with Ran-GTP and the complex moves to the cytoplasm, where GTP is hydrolyzed to GDP and the cargo is released. For import, the RNA and its importin receptor cross through a nuclear pore into the nucleus, where GDP is exchanged for GTP and the RNA cargo is released.
For noncoding RNAs that are processed in the cytoplasm and returned to the nucleus, the cargo RNA and its import receptor—the importin—cross through a nuclear pore into the nucleus, where the cargo is released as the importin binds to Ran-GTP. The importin-Ran-GTP then translocates back to the cytoplasm, the importin dissociates, and GTP is hydrolyzed to GDP, restarting the cycle. Thus, export and import are reverse processes for which directionality is maintained by the presence of Ran-GTP in the nucleus and Ran-GDP in the cytoplasm.
Spliced mRNA crosses through a nuclear pore via a Ran-independent pathway, involving a different set of export factors that associate with other proteins to form a much larger complex called TREX (transcription-export). TREX joins the machineries responsible for the transcription, splicing, and export of mRNA. The TREX complex is specifically recruited to actively transcribed genes through interactions with Pol II. In human cells, the TREX component protein Aly recruits the complex to the 5′ cap of nascent transcripts by interacting with the cap-binding protein CBP80. Because TREX functions as a nuclear export factor, mRNAs are transported out of the nucleus in the 5′→3′ direction through a nuclear pore.
mRNA Export from the Nucleus Is Coupled to Pre-mRNA Splicing
In eukaryotes, pre-mRNA splicing necessarily precedes export of the mature transcript from the nucleus, and researchers wondered whether pre-mRNA processing and nuclear export were closely coupled—which would provide a mechanism of mRNA quality control. To test this possibility, either pre-mRNA containing a single intron or the same mRNA lacking the intron were injected into the nuclei of frog eggs. The rate of nuclear export of each type of mRNA was then measured by detecting the presence of the RNA in the cytoplasm over time. Results of this experiment showed that intron-containing pre-mRNAs, which were spliced in the nucleus, were exported much more rapidly and efficiently than the identical mRNAs lacking the intron. Furthermore, the spliced mRNA was found to assemble with a different set of proteins than the mRNA lacking the intron. This led to the conclusion that splicing generates a specific mRNA-protein complex that targets the mRNA for nuclear export, explaining the broader observation that an intron is required for the efficient expression of many eukaryotic protein-coding genes. In this way, only those mRNAs that have the correct end structures and spliced-exon sequence are used for protein synthesis.
Mature mRNAs produced by splicing end up in different intracellular locations and are differently translated and degraded than otherwise identical mRNAs produced from non-intron-containing genes. The explanation is that splicing influences the set of proteins that associate with the mRNA in the nucleus to form an mRNP (mRNA ribonucleoprotein particle). These proteins, in turn, ensure that the mRNA interacts with exportins for shipment out of the nucleus. Chemical cross-linking experiments with cell extracts translating mRNAs with or without introns showed that several proteins bind to exon-exon junctions only as a consequence of splicing. Spliceosomes deposit a complex of proteins called the exon junction complex (EJC) on mRNAs at a position 20 to 24 nucleotides upstream of exon-exon junctions (Figure 16-23). At its core, EJC contains four proteins: eIF4AIII, MAGOH, Y14, and MLN51. The bound complexes accompany spliced mRNA into the cytoplasm, where they are removed during the first (“pioneer”) round of translation.
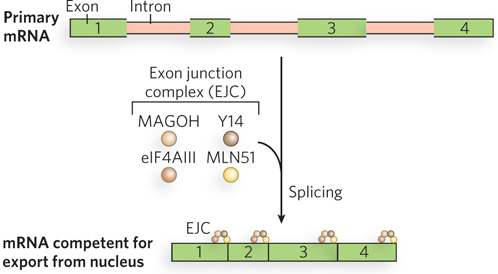
Figure 16-23: The exon junction complex. The EJC is a complex of four proteins responsible for mRNA quality control. An EJC is deposited on the mRNA after splicing, just upstream of each exon-exon junction. The complexes accompany the mature mRNA out of the nucleus and into the cytoplasm.
Some mRNAs Are Localized to Specific Regions of the Cytoplasm
In specialized cells, including oocytes (egg cells) and neurons, mRNAs are localized to particular sites prior to translation. The mechanism of such mRNA localization is best characterized in the fruit fly Drosophila melanogaster, in which maternal mRNAs are trafficked to various parts of the egg to help establish polarity during the early stages of embryo development (Figure 16-24). Shortly after the egg begins to mature, but before fertilization, mRNAs encoding proteins called Oskar and Bicoid bind to proteins that can move along microtubules, filamentous protein polymers that contribute to cell shape and structure. The oskar and bicoid mRNAs are shuttled to the parts of the egg where their protein products are required to form structures in the developing embryo. Analogous mechanisms of mRNA localization are thought to occur in neurons, in which mRNAs must be moved to parts of the cell very far from the nucleus for the localized protein synthesis required for proper neural function.
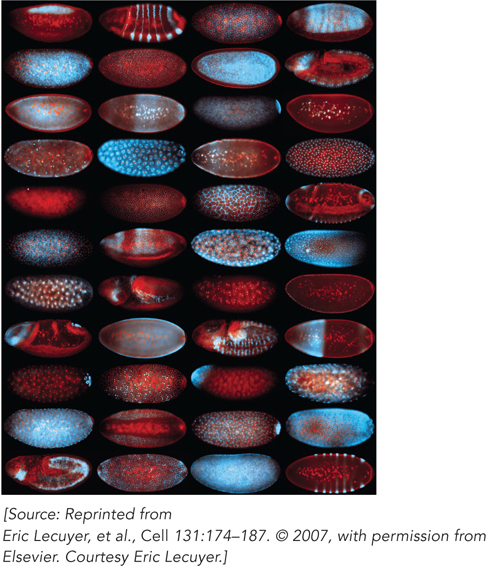
Figure 16-24: Transport of mRNA in Drosophila eggs and embryos. The building blocks of anterior-posterior axis patterning in Drosophila are laid out during egg formation (oogenesis), well before the egg is fertilized and deposited. The developing egg (oocyte) is polarized by differentially localized mRNA molecules. In each oocyte shown, a different mRNA is labeled with a blue fluorescent marker. Nuclear DNA is labeled with a red marker. Each mRNA contributes to pattern formation in the developing embryo.
An obvious advantage of regulating gene expression by mRNA localization is that it allows protein production to be spatially restricted within the cytoplasm. In this way, production can be turned on (and off) as required, without waiting for transcription, mRNA export, translation, and subsequent targeting of the protein to the site where it is needed. In addition, localized mRNAs can be translated multiple times to generate many copies of a protein at the required site. Local translation can also protect the rest of the cell from proteins that would be toxic or interfere with functions in other cellular compartments.
Cellular mRNAs Are Degraded at Different Rates
As for all molecules in the cell, the amount of an mRNA transcript is determined by its relative rates of synthesis and degradation, or decay. Cellular mRNAs are degraded as part of their normal life cycle. RNA degradation, catalyzed by ribonucleases, is the complete hydrolysis of RNA molecules into their component nucleotides. When mRNA synthesis and decay rates are balanced, the concentration of the mRNA remains in a steady state. A change in either rate will lead to a net accumulation or depletion of the mRNA, affecting the rate of protein synthesis. Degradative pathways ensure that mRNAs do not build up in the cell and direct the synthesis of unnecessary proteins.
In eukaryotic cells, degradation rates for mRNAs produced from different genes can vary greatly, from a half-life of minutes or even seconds for a gene product that is needed only briefly, to many cell generations for a gene product in constant demand. Bacterial cells grow much faster than eukaryotic cells and must rapidly adapt to changing environmental and metabolic conditions; their mRNAs are stable for only a few minutes. Degradation rates of an RNA are affected by its primary and secondary structure. For instance, a hairpin structure in bacterial and eukaryotic mRNAs can confer stability. In eukaryotes, sequences rich in A and U residues, known as AU-rich elements (AREs), occur in the 3′UTRs of some mRNAs. These elements recruit factors such as nucleases or RNA-binding proteins that can enhance or reduce, respectively, the degradation rate of the mRNA.
For mRNAs, degradation in E. coli begins with one or a few cuts by an endoribonuclease, followed by 3′→5′ degradation by an exoribonuclease. In eukaryotes, the poly(A) tail is shortened, the 5′ cap is removed, then the mRNA can be degraded by ribonucleases. Eukaryotes have a complex of up to 10 conserved 3′→5′ exoribonucleases, called the exosome, which, besides degrading mRNAs, is involved in the processing of the 3′ end of rRNAs and tRNAs (Figure 16-25). The exosome is the major path of mRNA degradation in higher eukaryotes, but lower eukaryotes use primarily 5′→3′ exonucleases.
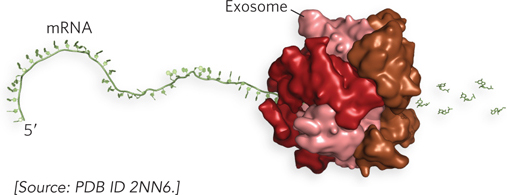
Figure 16-25: The human exosome. The exosome has a ringlike structure. The complex encircles the mRNA and slides along in a 3′→5′ direction, degrading the RNA as it moves.
Decay rates and half-lives are determined by proteins that enhance or inhibit exosome binding through interactions with the 3′UTR of the mRNA. In addition, two mRNA surveillance pathways are in place to respond to mRNAs that contain a premature stop codon or lack a stop codon. Nonsense-mediated decay (NMD) is triggered by exon junction complexes, which, as we have seen, are deposited during pre-mRNA splicing in the nucleus. Normally, EJCs are removed by the ribosome during the first round of translation, but if an EJC is located downstream of a stop codon, its presence indicates that splicing occurred at this position and that there should be additional coding sequence after the stop codon. The EJC thus signals that this is an mRNA with a premature stop codon, or nonsense codon, and triggers mRNA degradation. In contrast, the non-stop decay pathway targets mRNA molecules lacking a stop codon. Ribosomes traversing these mRNAs are released from the 3′ end of the message, and the mRNA is shunted to the exosome for degradation.
Processing Bodies Are the Sites of mRNA Storage and Degradation in Eukaryotic Cells
In eukaryotes, mRNAs that are not engaged in translation are sequestered in localized areas of the cytoplasm called processing bodies (P bodies), which can be observed by light microscopy. P bodies contain proteins that catalyze removal of the mRNA 5′ cap and thus are thought to be sites of mRNA degradation. They also seem to be sites where mRNAs are temporarily stored when not being translated and may therefore play an active role in regulating which proteins are made in response to the cell’s needs (Figure 16-26).
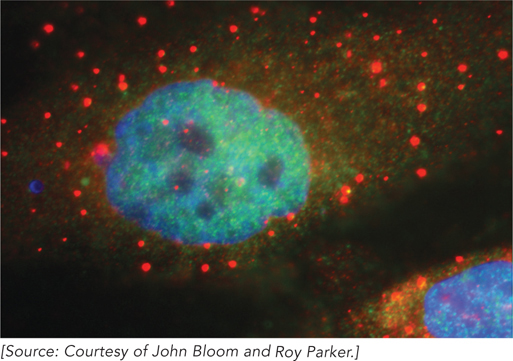
Figure 16-26: Processing (P) bodies in human cells. In these HeLa cells, P bodies (red spots) are visualized by staining with an antibody that binds to a protein involved in mRNA turnover. Nuclei are stained blue.
Recent experimental evidence supports an emerging model of cytoplasmic mRNA function in which translation and degradation rates are influenced by the relative concentrations of mRNA in polyribosomes (groups of ribosomes translating an mRNA; see Chapter 18) and in P bodies. In some cases, mRNA-specific binding factors suppress translation and promote degradation by recruiting P-body proteins to individual mRNAs. Notably, many of the proteins necessary for microRNA (miRNA) gene silencing (discussed in Chapter 22) are localized to P bodies, including the scaffold protein GW182, Argonaute (Ago), 5′ decapping enzymes, and helicases (enzymes that unwind RNA). The current evidence suggests that P bodies are scaffolding centers of miRNA function, especially given the finding that reduction of GW182 levels disrupts P-body formation.
SECTION 16.3 SUMMARY
Non-protein-coding RNAs are exported from the nucleus by exportin proteins in a Ran-dependent pathway that requires GTP.
After splicing, eukaryotic mRNAs are recognized by their processing modifications and then exported to the cytoplasm through a nuclear pore.
Spliced mRNA exits the nucleus in a Ran-independent pathway involving export factors and other proteins in a large complex called TREX, which coordinates the machineries responsible for transcription, splicing, and export of mRNA.
Spliceosomes deposit a complex of proteins—the exon junction complex (EJC)—on spliced mRNAs upstream from exon-exon junctions, providing a physical tag indicating that splicing has occurred. The EJC promotes nuclear export and mRNA stability.
Cellular mRNAs are degraded at different rates, depending on their interactions with ribonucleases. Nonsense-mediated decay and non-stop decay are two pathways that guard against the translation of defective mRNAs.
Bacterial mRNA degradation begins with one or a few cuts by an endoribonuclease, followed by 3′→5′ degradation by an exoribonuclease.
In eukaryotes, a complex of up to 10 conserved 3′→5′ exoribonucleases—the exosome—helps degrade mRNAs and processes the 3′ end of rRNAs and tRNAs.
Processing bodies (P bodies) are cytoplasmic locations of mRNA storage and degradation.




