18.2 ACTIVATION OF AMINO ACIDS FOR PROTEIN SYNTHESIS
For the synthesis of a polypeptide with a sequence defined by an mRNA, two chemical requirements must be met: (1) the carboxyl group of each amino acid must be activated to facilitate the formation of a peptide bond, and (2) a link must be established between each new amino acid and the information in the mRNA that encodes it. Both of these are satisfied by covalent attachment of the amino acid to a tRNA prior to protein synthesis. Attaching each amino acid to its corresponding tRNA is critical. This reaction takes place in the cytosol, not on the ribosome. Each of the 20 common amino acids is covalently linked to a specific tRNA at the expense of ATP hydrolysis, catalyzed by Mg2+-dependent activating enzymes known as aminoacyl-tRNA synthetases. When attached to an amino acid, a tRNA is said to be charged.
Amino Acids Are Activated and Linked to Specific tRNAs
The charging of a tRNA requires the formation of an acyl linkage between the carboxyl group of an amino acid and the free 2′- or 3′-hydroxyl end of the tRNA. All tRNAs share a similar structure, including three or four arms and a 3′-terminal CCA sequence (see Figures 6-22 and 17-2). The acylation reaction results in attachment of the amino acid to the 3′-terminal adenosine.
Aminoacyl-tRNA synthetases must activate the amino acid before it is attached to the tRNA. This reaction occurs in two steps in the enzyme’s active site. In the adenylylation step (Figure 18-11, step 1), an enzyme-bound intermediate, 5′-aminoacyl adenylate (5′-aminoacyl-AMP), forms when the carboxyl group of the amino acid reacts with the α-phosphoryl group of ATP to form a phosphoanhydride linkage, with displacement of pyrophosphate. In the subsequent tRNA-charging step (step 2), the aminoacyl group is transferred from enzyme-bound aminoacyl-AMP to its specific tRNA. The amino acid can be transferred to either the 2′-OH or the 3′-OH (left and right paths, respectively, in Figure 18-11) of the 3′-terminal adenosine of the tRNA, depending on the type of aminoacyl-tRNA synthetase. Class I synthetases attach the amino acid to the 2′-OH, and class II synthetases attach the amino acid to the 3′-OH. In the class I pathway, the aminoacyl ester linkage migrates to the 3′-OH position spontaneously by a transesterification reaction.
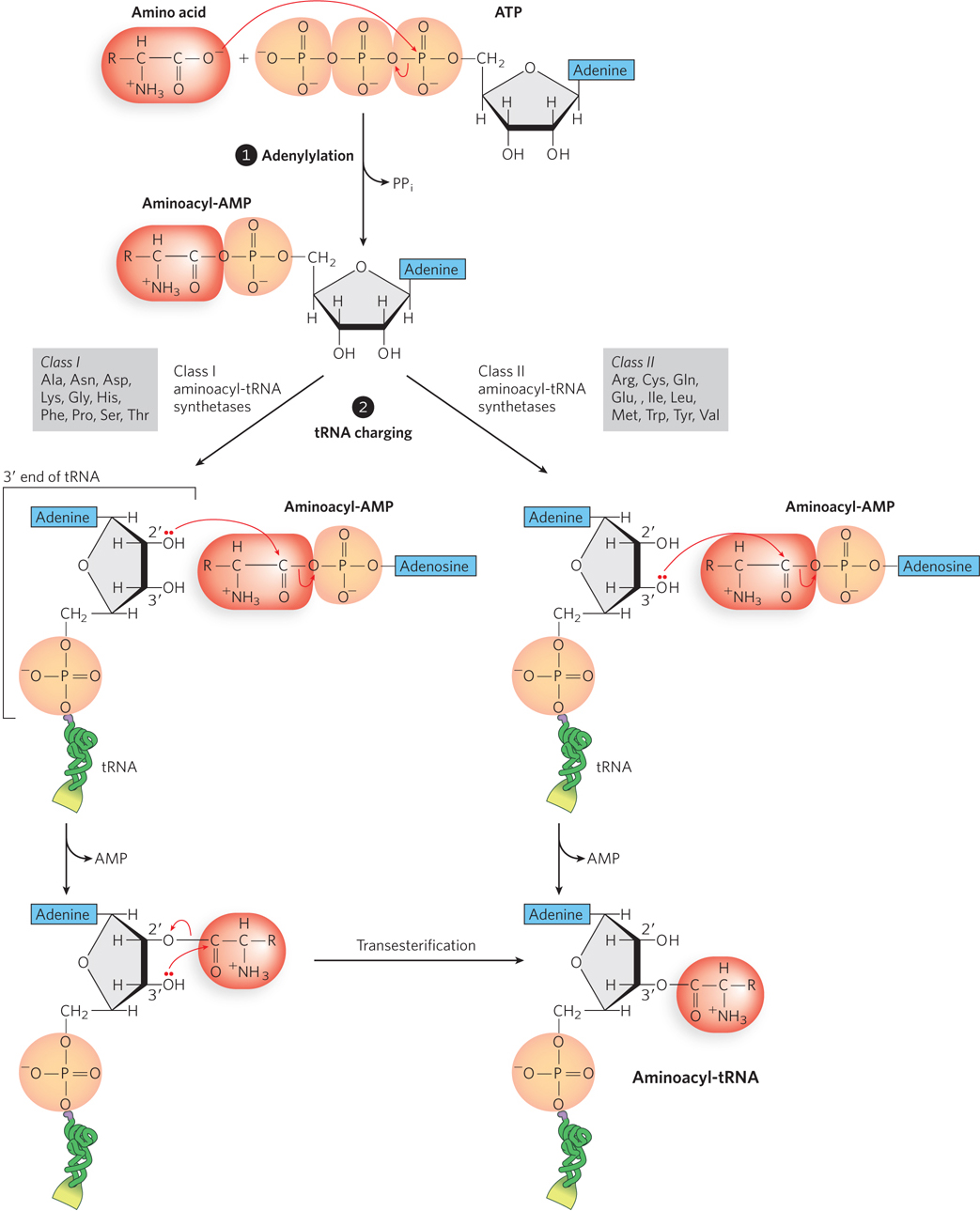
Figure 18-11: Charging of tRNAs by aminoacyl-tRNA synthetases. In step 1, the adenylylation step, the amino acid is linked to adenylate, forming aminoacyl-AMP. In step 2, the amino acid is transferred from the AMP to the tRNA in one of two pathways, catalyzed by a class I or class II aminoacyl-tRNA synthetase. The synthetases in each class are listed (denoted by the amino acid they transfer to tRNA).
The resulting ester linkage between the amino acid and the tRNA has a highly negative standard free energy of hydrolysis (ΔG′° = −229 kJ/mol). Because its hydrolysis is energetically favorable, this bond between the amino acid and the tRNA provides an energetic driving force for translation. The first part of the activation reaction separates two phosphates from ATP instead of just one (as in many other ATP-driven reactions). Ultimately, the energy stored in the bond between the two phosphates is released on hydrolysis by the enzyme inorganic pyrophosphatase. Thus, two high-energy phosphate bonds are ultimately expended for each amino acid molecule activated, rendering the overall reaction for amino acid activation essentially irreversible.
Aminoacyl-tRNA Synthetases Attach the Correct Amino Acids to Their tRNAs
A distinct aminoacyl-tRNA synthetase is responsible for attaching each of the 20 common amino acids to its corresponding tRNA. Each enzyme is specific for one amino acid but can recognize more than one tRNA, because most amino acids are specified by more than one codon (as discussed in Chapter 17). The structures of all the aminoacyl-tRNA synthetases of E. coli have been determined, and the structures reflect their mode of tRNA recognition and catalytic activity (Figure 18-12). Class I enzymes are typically monomeric and attach the amino acid to the 2′-OH of the 3′-terminal adenine of tRNA. As noted above, the aminoacyl group spontaneously migrates to the 3′-OH position. Class II aminoacyl-tRNA synthetases are sometimes multimeric, and they approach their tRNA substrate from a different side than the class I synthetases, typically attaching the amino acid to the 3′-OH. These two classes of aminoacyl-tRNA synthetases are the same in all organisms. There is no evidence for a common ancestor from which the two classes diverged, and the biological, chemical, or evolutionary reasons for two enzyme classes for essentially identical processes remain obscure.
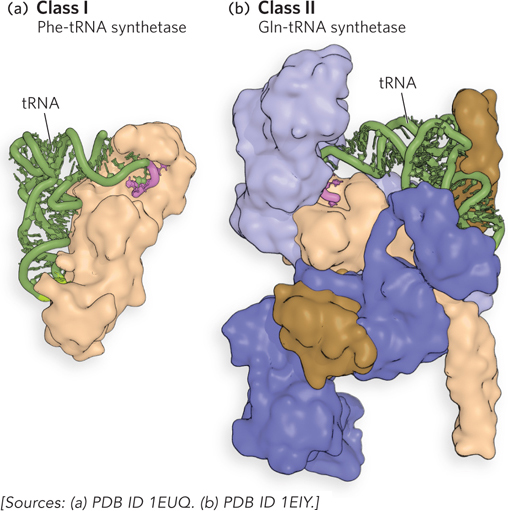
Figure 18-12: Crystal structures of aminoacyl-tRNA synthetases with bound tRNA. (a) Phe-tRNA synthetase is a class I and (b) Gln-tRNA synthetase is a class II aminoacyl-tRNA synthetase. For each structure, the bound tRNA is shown in green, and the 3′ end of the tRNA to which the amino acid attaches is shown in pink.
There are exceptions to the rule of one aminoacyl-tRNA synthetase for one amino acid. Because the synthetase sequences are evolutionarily related, they are usually readily identified in genomic sequence data, and the absence of a gene encoding the aminoacyl-tRNA synthetase specific for glutamine in some bacteria was initially puzzling. Biochemical and genetic experiments revealed that in these bacteria, a single enzyme charges both tRNAGln and tRNAGlu with glutamate. A second enzyme then catalyzes an amination reaction that converts the glutamate of Glu-tRNAGln to glutamine.
The Structure of tRNA Allows Accurate Recognition by tRNA Synthetases
The overall fidelity of protein synthesis requires that each individual aminoacyl-tRNA synthetase must be specific for a single amino acid and for certain tRNAs. The interaction of aminoacyl-tRNA synthetases and tRNAs has been referred to as the “second genetic code,” reflecting its critical role in maintaining the accuracy of protein synthesis. The “coding” rules seem to be more complex than those of the “first” code.
By observing changes in nucleotides that alter substrate specificity, researchers have identified nucleotide positions that are involved in substrate discrimination by the aminoacyl-tRNA synthetases (Figure 18-13). Some nucleotides are conserved in all tRNAs and therefore cannot be used for discrimination. Although in some cases the nucleotides of the anticodon itself are recognized, nucleotide positions conferring synthetase specificity tend to be concentrated in the amino acid arm and elsewhere in the anticodon arm, as well as other parts of the tRNA molecule. Determination of the crystal structures of aminoacyl-tRNA synthetases complexed with their cognate tRNAs and ATP has added a great deal to our understanding of these interactions.
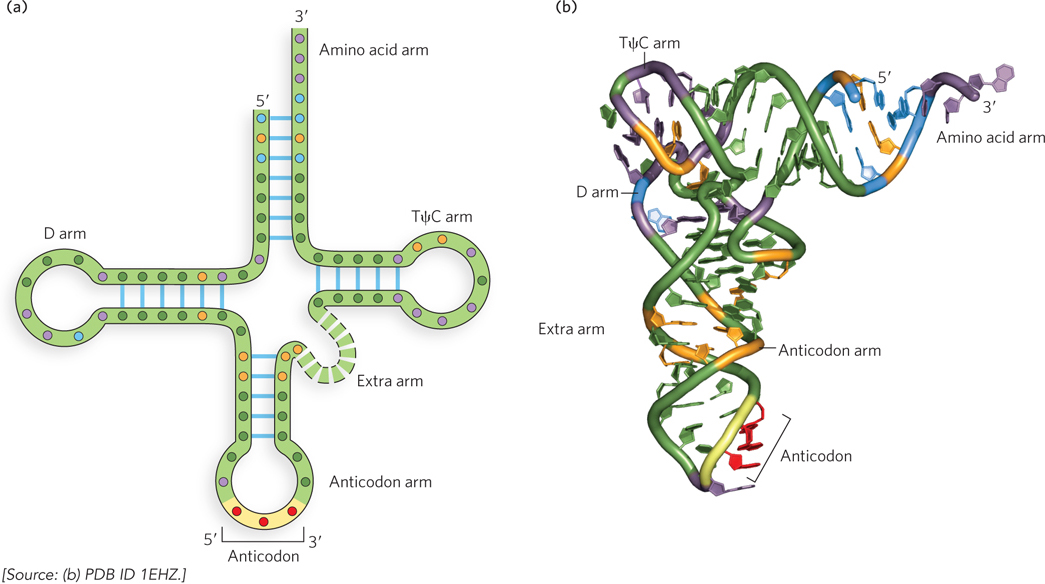
Figure 18-13: Sequence features of a tRNA that are recognized by aminoacyl-tRNA synthetases. (a) The two-dimensional and (b) three-dimensional structures of tRNA, showing the nucleotides recognized by only one (orange residues) or by two or more (blue residues) aminoacyl-tRNA synthetases. Nucleotides shown in purple are common to all tRNAs and therefore cannot be used to distinguish among them; nucleotides shown in black are positions in the tRNA sequence that are not specificity determinants.
Ten or more specific nucleotides may be involved in the recognition of a tRNA by its specific aminoacyl-tRNA synthetase. But in a few cases, the recognition mechanism is quite simple. For example, for alanyl-tRNA synthetase (or Ala-tRNA synthetase—a shorthand commonly used for these enzymes), across a range of organisms from bacteria to humans, the primary determinant of recognition of tRNAAla is a single G–U base pair in its amino acid arm. A short RNA with as few as 7 bp arranged in a simple hairpin mini-helix is efficiently aminoacylated by the Ala-tRNA synthetase, as long as the RNA contains the critical G–U.
Proofreading Ensures the Fidelity of Aminoacyl-tRNA Synthetases
The aminoacylation of tRNA both activates an amino acid for peptide bond formation and appends the amino acid to an adaptor tRNA that ensures appropriate placement of the amino acid in a growing polypeptide. The identity of the amino acid attached to a tRNA is not checked on the ribosome, however, so attachment of the correct amino acid to the tRNA is essential to the fidelity of protein synthesis.
Discrimination between two similar amino acid substrates has been studied in detail in the case of Ile-tRNA synthetase, which must distinguish between valine and isoleucine, amino acids that differ by just a single methylene group (–CH2–). Because valine is smaller, Val-tRNA synthetase can discriminate between valine and isoleucine by having a binding pocket too small for isoleucine to bind (Figure 18-14a). However, the correlating strategy cannot work for Ile-tRNA synthetase, because the small valine molecule can fit in the big isoleucine pocket. Instead, Ile-tRNA synthetase must rely on the energetics of substrate binding and proofreading. Ile-tRNA synthetase favors activation of isoleucine (to form Ile-AMP) over valine by a factor of 200—as we might expect, given the amount by which a methylene group (in Ile) could enhance substrate binding. Yet a Val residue is erroneously incorporated into proteins in positions normally occupied by an Ile residue at a frequency of about 1 in 3,000. How is this more than tenfold increase in accuracy brought about? Ile-tRNA synthetase, like some other aminoacyl-tRNA synthetases, has a proofreading function.
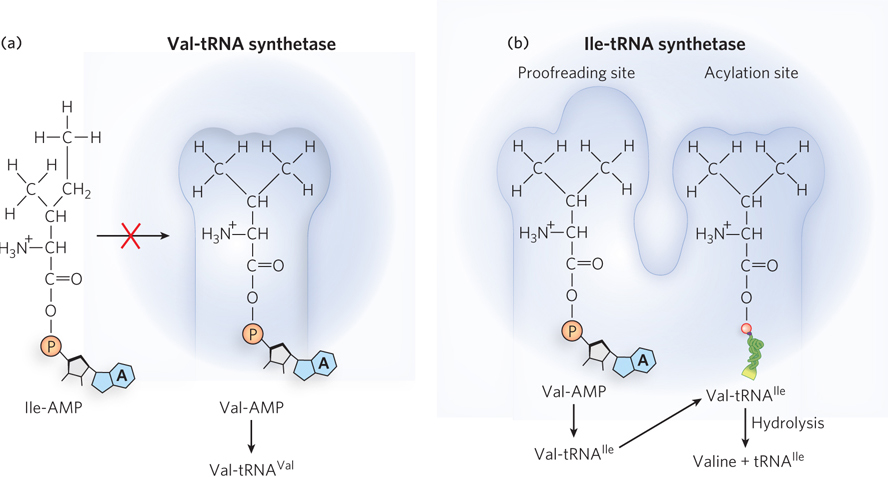
Figure 18-14: Proofreading by an aminoacyl-tRNA synthetase. (a) tRNAVal is charged in the acylation site of the Val-tRNA synthetase. Isoleucine is larger than valine and does not fit in the acylation site. (b) tRNAIle is charged in the acylation site of the Ile-tRNA synthetase. Because valine is smaller than isoleucine, tRNAIle is sometimes charged with valine. This incorrectly charged Val-tRNAIle fits into the synthetase’s proofreading site, where it is hydrolyzed to release valine from the tRNA.
A general principle of proofreading by enzymes is that if available binding interactions do not provide sufficient discrimination between two substrates, the necessary specificity can be achieved by substrate-specific binding in two successive steps. The effect of forcing the system through two consecutive filters is multiplicative. In the case of Ile-tRNA synthetase, the first filter is the initial binding and activation of the amino acid to form the aminoacyl-AMP, followed by transfer of the amino acid to the tRNA. The second is the binding of any incorrect aminoacyl-tRNA products to a separate active site on the enzyme; a substrate that binds in this second active site is hydrolyzed (Figure 18-14b). Because the R group of valine is slightly smaller than that of isoleucine, Val-tRNA fits the hydrolytic (proofreading) site of the Ile-tRNA synthetase but Ile-tRNA does not. Thus, only Val-tRNA is hydrolyzed in the proofreading active site.
The greatly accelerated rate of hydrolysis of incorrectly charged tRNAs provides an important mechanism for enhancing the fidelity of the overall process. This is an example of kinetic proofreading, in which a complex process occurs in multiple steps, the rates of which are tuned to maximize the speed of correct reactions while stalling and reversing incorrect reactions. Note that this proofreading mechanism requires energy, because it requires a second round of aminoacylation (with the correct amino acid). The few aminoacyl-tRNA synthetases that activate amino acids with no close structural relatives (e.g., Cys-tRNA synthetase) demonstrate little or no proofreading activity; in these cases, the active site for aminoacylation can sufficiently discriminate between the proper substrate and any other, incorrect amino acid.
In summary, translation relies on aminoacyl-tRNA synthetases to ensure the correct charging of tRNAs, because the ribosome does not distinguish between correctly and incorrectly charged tRNAs during protein synthesis. The decoding center of the ribosome is designed to detect and favor proper codon-anticodon base pairing, but does not link this information to the identity of the amino acid in the peptidyl transferase center at the other end of the tRNA (see the How We Know section at the end of this chapter). As a result, the overall error rate of protein synthesis (∼1 mistake per 104 amino acids incorporated) is significantly higher than that of DNA replication. Modern protein technology has been designed to exploit this lack of proofreading so that ribosomes can incorporate synthetic amino acids into proteins, as described in Highlight 18-2. In cells, flaws in a protein are eliminated when the protein is degraded and are not passed on to future generations, so they have less biological significance than errors in DNA. The degree of fidelity in protein synthesis is sufficient to ensure that most proteins contain no mistakes and that the large amount of energy required to synthesize a protein is rarely wasted. One defective protein molecule is usually unimportant when the cell contains many correct copies of the same protein.
HIGHLIGHT 18-2 TECHNOLOGY: Genetic Incorporation of Unnatural Amino Acids into Proteins

Peter Schultz
Peter Schultz and his colleagues at the Scripps Research Institute wondered whether the specificity of aminoacyl-tRNA synthetases, together with the lack of discrimination of mischarged tRNAs by ribosomes, could be exploited to incorporate unnatural amino acids into cellular proteins. First they introduced stop codons at sites in an mRNA where they hoped to introduce an unnatural amino acid in the corresponding polypeptide. Next they engineered suppressor tRNAs (see Chapter 17) with an anticodon sequence complementary to the stop codon. Taking advantage of the known structural determinants of the synthetases’ recognition of tRNA, the researchers designed suppressor tRNAs to be charged by aminoacyl-tRNA synthetases with mutations that caused them to use unnatural amino acid substrates (Figure 1). Schultz’s research group showed that bacterial, yeast, and mammalian cells containing the engineered mRNAs, tRNAs, and aminoacyl-tRNA synthetases, along with unnatural amino acids, would produce proteins with the unnatural amino acid residues at the planned positions.
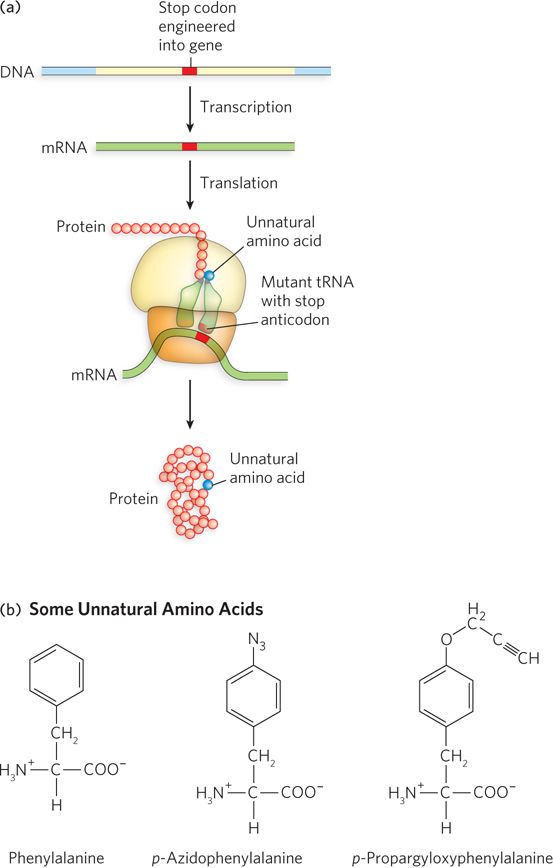
FIGURE 1 (a) An mRNA stop codon and a mutated tRNA (with a stop anticodon) are used to introduce unnatural amino acids into proteins. (b) Two unnatural amino acids, derivatives of phenylalanine (also shown, for comparison), that can be incorporated by this method.
This method allows the incorporation into proteins of fluorescent, glycosylated, sulfated, metal ion–binding, and redox-active (i.e., electron-transferring) amino acids, as well as amino acids with new chemical and photochemical reactivity. Why might such technology be useful? Schultz hopes to use the approach to explore protein structure and function, both in vitro and in vivo, by incorporating chemical tags or probes that can report on their local molecular/structural environment or provide molecular “beacons” in cells. Furthermore, with the ability to generate proteins with new or enhanced properties by making use of amino acids not found in nature, it may eventually be possible to enable cells to synthesize therapeutic proteins.
SECTION 18.2 SUMMARY
Aminoacyl-tRNA synthetases covalently link amino acids to tRNAs to create the substrates used by the ribosome during protein synthesis. The acylation reaction results in a high-energy bond that supplies the energetic driving force for translation.
A different aminoacyl-tRNA synthetase exists for each amino acid. Because multiple codons can specify a single amino acid, most aminoacyl-tRNA synthetases can recognize multiple tRNAs bearing anticodons complementary to the codons for a particular amino acid.
The anticodon is responsible for the specificity of interaction between the aminoacyl-tRNA and the complementary mRNA codon, but it rarely provides the primary site for synthetase recognition.
Two successive binding steps allow kinetic proofreading that increases the fidelity of tRNA aminoacylation. This is essential because ribosomes do not discriminate between correctly and incorrectly charged tRNAs during protein synthesis.





