22.5 PUTTING IT ALL TOGETHER: GENE REGULATION IN DEVELOPMENT
The patterns of gene regulation that bring about development from a zygote to a multicellular animal or plant are complex and intricately coordinated. Development requires transitions in protein composition and in morphology that depend on tightly coordinated changes in expression of the genome.
How is a complex organism produced, with its many tissues and organs and appendages, from a single cell? Some clues can be found in that single cell—
The regulatory mechanisms used in development encompass all of the regulatory processes discussed in Chapter 21 and in this chapter thus far. Transcriptional regulation occurs, but posttranscriptional regulatory processes are particularly important.
Development Depends on Asymmetric Cell Divisions and Cell-Cell Signaling
If all cells divided to produce two identical daughter cells, multicellular organisms could never be more than a ball of identical cells. Programmed asymmetric cell divisions are required for different cell fates. Cell-
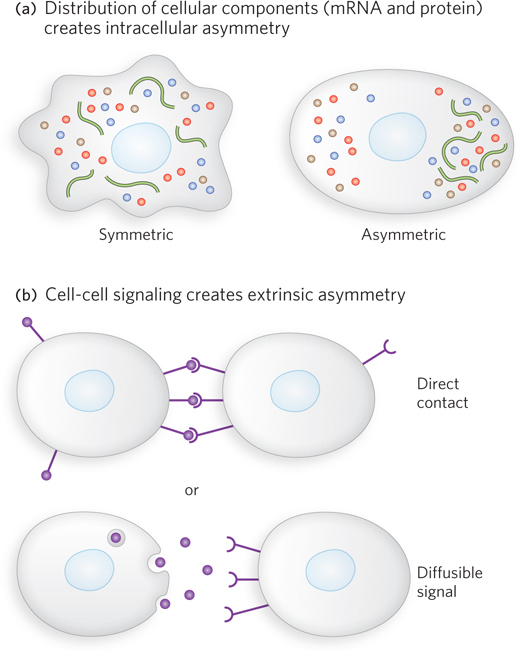
Asymmetry within the cells themselves takes the form of gradients of mRNAs and proteins that define critical axes (posterior-
It is not enough to create a gradient in the cell, however. The mitotic spindle must also be aligned along the same axis as the gradient, so that the cell division occurs on an axis perpendicular to the gradient (see Chapter 2 for a reminder about mitotic cell division). Ensuring the proper alignment of the mitotic spindle in particular cell divisions is the function of some proteins critical to development.
In the developing embryo, cell-
782
Characteristic Stages of Development Several organisms became important model systems for the study of development because they are easy to maintain in a laboratory and have relatively short generation times. These include nematodes, fruit flies, zebra fish, mice, and the plant Arabidopsis thaliana (see the Model Organisms Appendix). The discussion here focuses on developmental pathways in the fruit fly. Our understanding of the molecular events during development of Drosophila melanogaster is especially well advanced and can be used to illustrate patterns and principles of general significance, and highlight the mechanisms of gene regulation that govern this complex process.
Multicellular eukaryotes develop in a process that begins with the union of an egg and a sperm cell by fertilization, to create a zygote. The egg cell has been preprogrammed by the deposition of maternal mRNAs in gradients, such that concentrations of certain maternal mRNAs vary greatly from one end of the oocyte to the other. On fertilization, cell division begins. Early in development, the fate of particular cells is determined by the concentration of maternal mRNAs, as well as by the actions of regulatory genes. As development proceeds, cascades of regulatory genes guide the various cell lineages as different tissue types develop. Although the regulatory genes are numerous, they generally fall into a small number of classes that are highly conserved, from nematodes to fruit flies to humans. Signaling pathways and processes are also highly conserved.
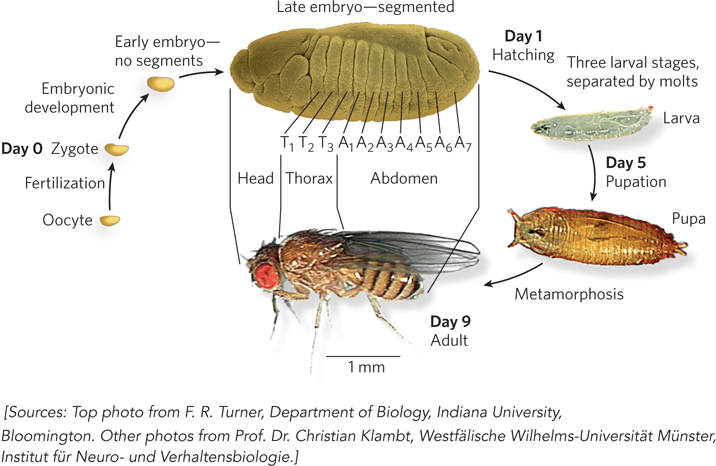
The life cycle of the fruit fly is relatively complex, and the patterns are conserved in a wide range of multicellular eukaryotes. Complete metamorphosis occurs during progression from embryo to adult fly (Figure 22-22). The final structure of the adult is forecast by features that are evident in the embryo at a very early stage. One of the most important characteristics of the embryo is its polarity: the anterior and posterior ends of the animal are readily distinguished, as are its dorsal and ventral surfaces. The fly embryo also exhibits the key characteristic of metamerism, division of the body into serially repeating segments, each with characteristic features. During development, these segments become organized into head, thorax, and abdomen. Each segment of the adult thorax has a different set of appendages. The development of this complex pattern is genetically controlled, and pattern-
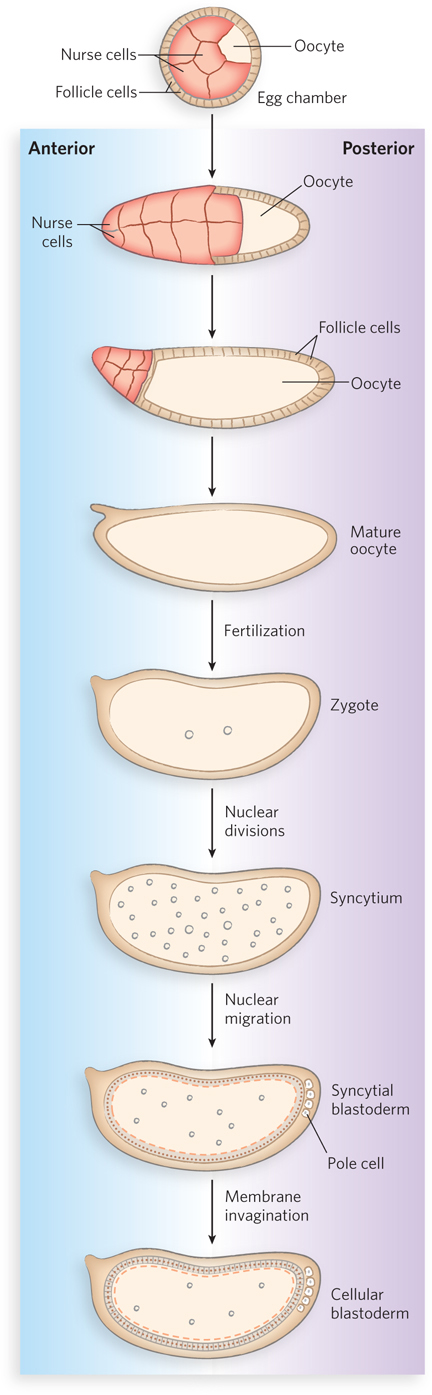
The Drosophila egg, with its 15 nurse cells, is surrounded by a layer of follicle cells (Figure 22-23). As the oocyte matures (before fertilization), mRNAs and proteins originating in the nurse and follicle cells are deposited in the egg cell, where many play a crucial role in development. After the fertilized egg is laid, the nucleus divides and the nuclear descendants continue to divide in synchrony every 6 to 10 minutes. Plasma membranes are not formed around the nuclei, which are distributed within the egg cytoplasm, forming a syncytium. During rounds 8 to 11 of nuclear division, the nuclei migrate to the egg’s outer layer, forming a monolayer surrounding the common yolk-
783
Cascades of Regulatory Proteins in Development The role of key genes in development is to regulate other genes. Temporal and spatial regulation is critical to the gradual maturation of cells and tissues as cell divisions continue from embryo to adult. As each successive layer of regulatory genes is activated, the embryo acquires a finer specialization of cellular function.
Several types of RNAs and proteins in the early embryo, and proteins with essential roles in later stages of development, follow patterns widely conserved in multicellular eukaryotes. As defined by Christiane Nüsslein-
Maternal genes are expressed in the unfertilized egg, and the resulting maternal mRNAs remain dormant until fertilization. Maternal mRNAs provide most of the required proteins in the very early stages of development, and in fruit flies, this occurs until the cellular blastoderm forms. Some of the proteins encoded by maternal mRNAs direct the spatial organization of the developing embryo to establish its polarity. Segmentation genes, transcribed after fertilization, direct the formation of the proper number of body segments. In nematodes, similar genes guide the formation of specific tissues following completion of the earliest stages of embryogenesis. At least three subclasses of segmentation genes act at successive stages of Drosophila development. Gap genes divide the developing embryo into several broad regions, and pair-
784
The many regulatory genes in these three classes direct the development of an adult organism, with a head, thorax, and abdomen, the proper number of segments, and the correct appendages on each segment. Although fruit fly embryogenesis takes about a day to complete, all these genes are activated during the first 4 hours. During this period, some mRNAs and proteins are present for only a few minutes at specific points in time. Some of the genes code for transcription factors that affect the expression of other genes in a kind of developmental cascade. Regulation at the level of translation also occurs, and many of the regulatory genes encode translational repressors, most of which bind to the 3′UTR of mRNAs. Because many mRNAs are deposited in the egg long before their translation is required, translational repression is especially important for regulation in developmental pathways.
Early Development Is Mediated by Maternal Genes
In invertebrates, a prescribed developmental path is evident from the very first embryonic cell division. The nonequivalence of the daughter cells of this first division implies a structural and functional asymmetry in the fertilized egg. The asymmetry is mediated by established gradients of molecules called morphogens—
In Drosophila, some maternal genes are expressed within the nurse and follicle cells, and some in the egg itself. In the unfertilized egg, the maternal gene products establish the critical anterior-
The anterior-
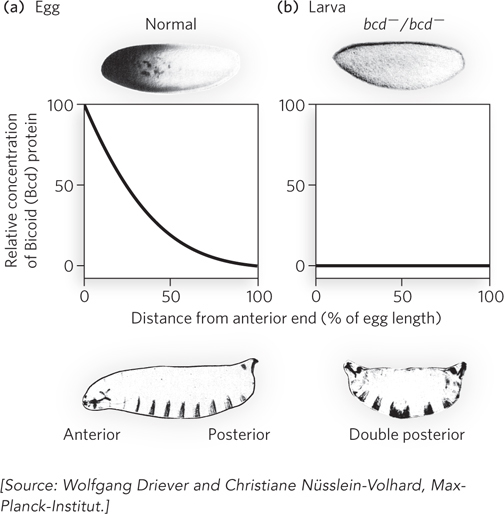
Bicoid contains a homeodomain (see Chapter 19). As a transcription activator, Bicoid activates the expression of several segmentation genes. It is also a translational repressor that inactivates certain mRNAs. The amounts of Bicoid in various parts of the embryo affect the subsequent expression of other genes in a threshold-
The nanos gene has an analogous role, but its mRNA is deposited at the posterior end of the egg, and the anterior-
785
A broader view of the effects of maternal genes in Drosophila reveals a more precise picture of a developmental circuit. In addition to bicoid and nanos mRNAs, deposited in the egg asymmetrically, several other maternal mRNAs are deposited uniformly throughout the egg cytoplasm. Three of them encode the Pumilio, Hunchback, and Caudal proteins—
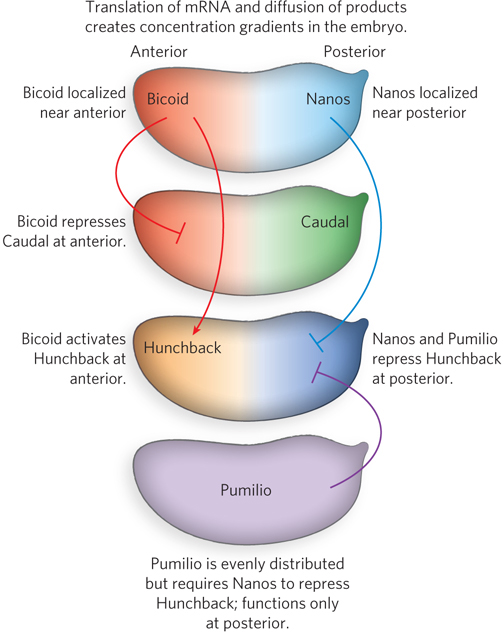
Caudal and Pumilio are involved in the development of the fruit fly’s posterior end. Caudal is a transcription activator with a homeodomain; Pumilio is a translational repressor from the PUF family of proteins (see Figure 22-28). Hunchback plays an important part in developing the anterior end; it is also a transcription factor for several genes, in some cases an activator and in others a repressor. Bicoid suppresses the translation of caudal mRNA at the anterior end and also acts as a transcription activator of hunchback mRNA in the cellular blastoderm. Because hunchback is expressed through maternal mRNAs and from genes in the developing egg, it is considered a maternal as well as a segmentation gene. The result of Bicoid’s activities is an increased concentration of Hunchback at the anterior end of the egg. Nanos and Pumilio act as translational repressors of hunchback, suppressing synthesis of Hunchback near the posterior end of the egg. Pumilio does not function in the absence of Nanos, and the gradient of nanos expression confines the activity of both proteins to the posterior region. Translational repression of the hunchback gene leads to degradation of hunchback mRNA near the posterior end. However, a lack of Bicoid in the posterior leads to expression of caudal. In this way, the Hunchback and Caudal proteins become asymmetrically distributed in the egg.
Segmentation Genes Specify the Development of Body Segments and Tissues
Segmentation genes are the zygotic genes that take over after maternal genes. Many operate at the level of transcriptional regulation. Gap genes, pair-
Pair-
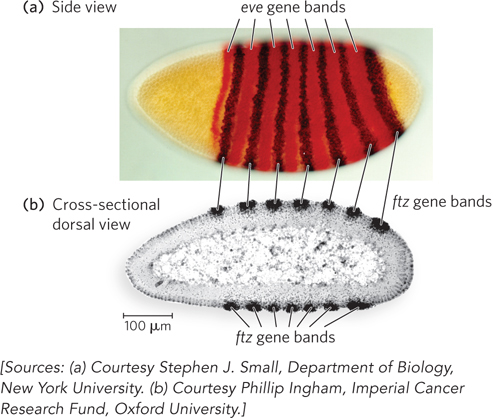
In the stripes where ftz is repressed, repression is mediated in part by another pair-
786
Gap and pair-
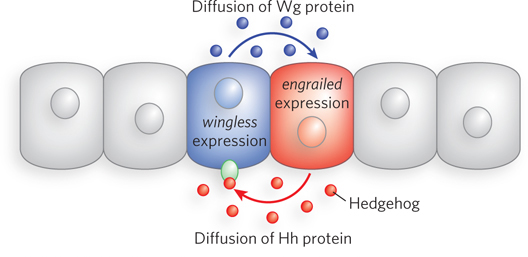
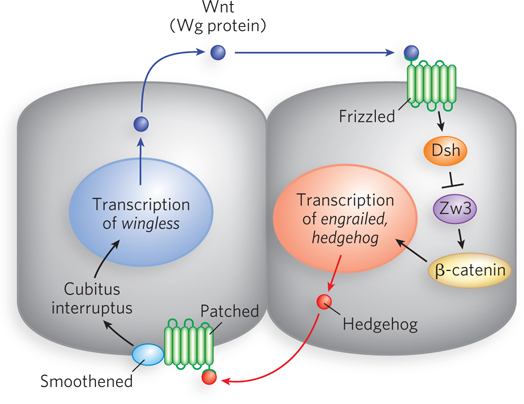
The Wg protein is a signal of the Wnt class, and studies of the wg gene helped define the Wnt-
787
Wnt proteins are highly homologous from one species to the next. They generally have a nearly invariant pattern of 23 Cys residues (some of which may form disulfide bonds needed for folding), an N-
The Wg protein is modified with lipids by an acyltransferase in the endoplasmic reticulum that is encoded by the gene porcupine. In nearby cells on the opposite side of the adjacent parasegment boundary, the secreted Wg protein interacts with a receptor that is the product of the gene frizzled (fz). In the recipient cell, the interaction triggers a signaling pathway that ultimately results in expression of the En protein. En is a transcription activator of the hedgehog gene. The Hedgehog (Hh) protein, part of a non–
Homeotic Genes Control the Development of Organs and Appendages
A set of 8 to 11 homeotic genes directs the formation of structures at specific locations in the body plan of most multicellular eukaryotes. Fewer homeotic genes are present in some simple eukaryotes. Even yeast has two homologs, regulators of mating-
Hox genes are sometimes organized in genomic clusters. Drosophila has one such cluster, and mammals have four. The order of genes within the clusters is colinear with their targets of action, from the anterior to the posterior of the developing embryo. In Drosophila, each Hox gene is expressed in a particular embryonic segment and controls the development of the corresponding part of the mature fly (Figure 22-29a). The terminology for describing Hox genes can be confusing. They have historical names in the fruit fly (e.g., ultrabithorax), whereas in mammals they are designated by two competing systems based on lettered (A, B, C, D) or numbered (1, 2, 3, 4) clusters (Figure 22-29b).
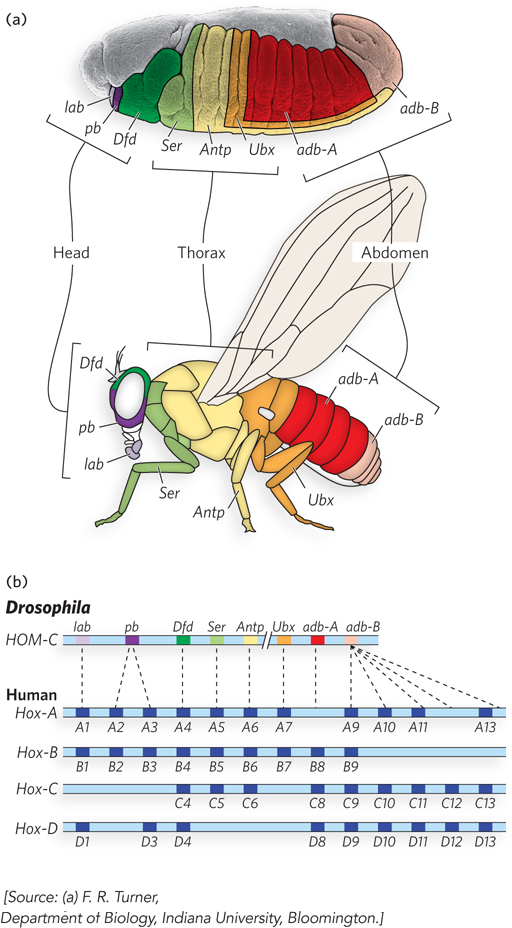
788
The loss of Hox genes in fruit flies, by mutation or deletion, causes the appearance of a normal appendage or body structure at an inappropriate body position. An important example is the ultrabithorax (ubx) gene. When the Ubx protein function is lost, the first abdominal segment develops incorrectly, with the structure of the third thoracic segment. Other known homeotic mutations cause the formation of an extra set of wings, or two legs at the position in the head where the antennae are normally found (Figure 22-30). The Hox genes often span long regions of DNA. The ubx gene, for example, is 77,000 bp long. More than 73,000 bp are in introns, one of which is more than 50,000 bp long. Transcription of the ubx gene takes nearly an hour. The delay this imposes on ubx gene expression is believed to be a timing mechanism involved in the temporal regulation of subsequent steps in development. Many Hox genes are further regulated by miRNAs encoded by intergenic regions of the Hox gene clusters. All Hox gene products are themselves transcription factors that regulate the expression of an array of downstream genes.
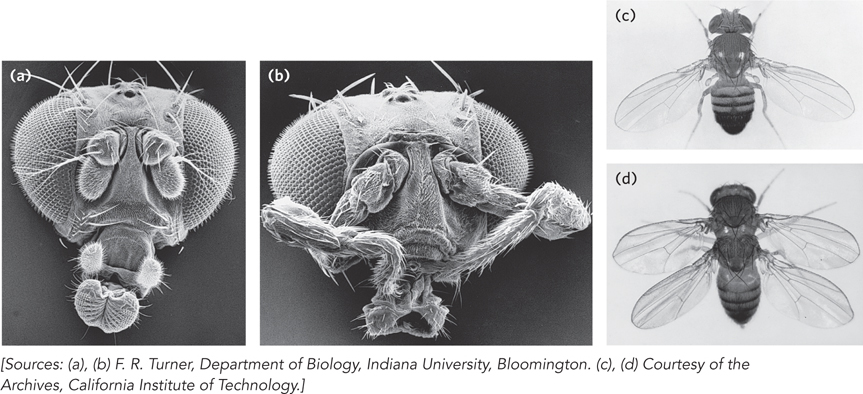
The conservation of some Hox genes is extraordinary. For example, the products of the homeobox-
Stem Cells Have Developmental Potential That Can Be Controlled
If we can understand development, and the mechanisms of gene regulation behind it, we can control it. An adult human has many different types of tissues. Many of the cells are terminally differentiated and no longer divide. If an organ malfunctions due to disease, or a limb is lost in an accident, the tissues are not readily replaced. Most cells, because of the regulatory processes that are in place, or even the loss of some or all genomic DNA, are not easily reprogrammed. Medical science has made organ transplants possible, but organ donors are a limited resource, and organ rejection remains a major medical problem. If humans could regenerate their own organs or limbs or nervous tissue, rejection would no longer be an issue. Real cures for kidney failure or neurodegenerative disorders could become reality.
The key to tissue regeneration lies in stem cells—cells that have retained the capacity to differentiate into various tissues. In humans, after an egg is fertilized, the first few cell divisions create a ball of totipotent cells (the morula), which have the capacity to differentiate individually into any tissue or even into a complete organism (Figure 22-31). Continued cell division produces a hollow ball, a blastocyst. The outer cells of the blastocyst eventually form the placenta. The inner layers form the germ layers of the developing fetus—
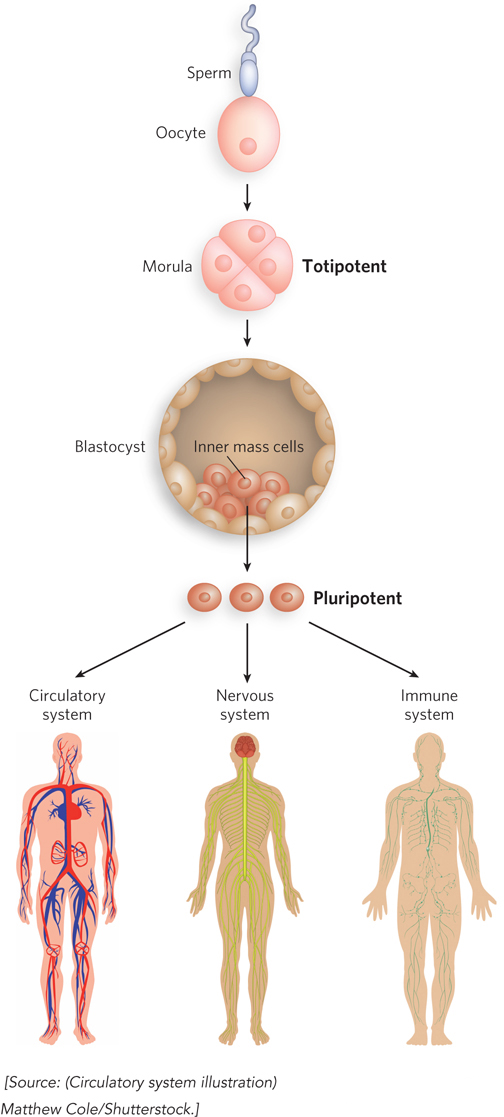
789
Stem cells have two functions: to replenish themselves and, at the same time, to provide cells that can differentiate. These tasks are accomplished in multiple ways (Figure 22-32a). All or parts of the stem cell population can, in principle, be involved in replenishment, differentiation, or both.
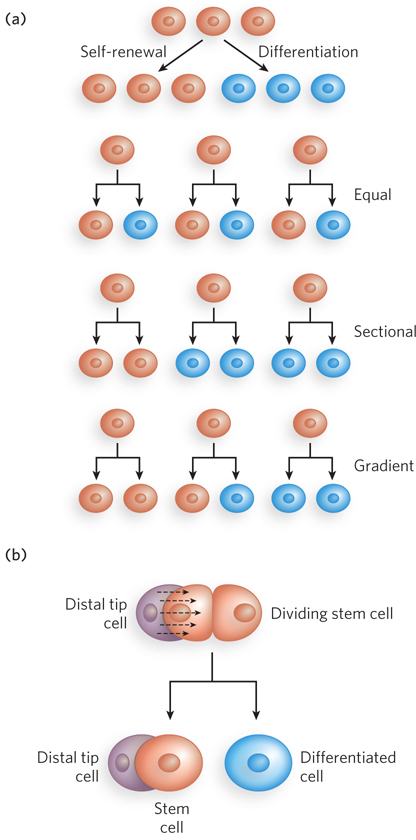
Other types of stem cells can potentially be used for medical benefit. In the adult organism, adult stem cells, as products of additional differentiation, have a more limited potential for further development than do embryonic stem cells. For example, the hematopoietic stem cells of bone marrow can give rise to many types of blood cells, as well as to cells with the capacity to regenerate bone. They are referred to as multipotent. However, these cells cannot differentiate into a liver or kidney or neuron. Adult stem cells are often said to have a niche, a microenvironment that promotes stem cell maintenance while allowing differentiation of some daughter cells as replacements for cells in the tissue they serve (Figure 22-32b). Hematopoietic stem cells in the bone marrow occupy a niche in which signaling from neighboring cells and other cues maintain the stem cell lineage. At the same time, some daughter cells differentiate to provide needed blood cells. Understanding the niche in which stem cells operate, and the signals the niche provides, is essential in efforts to harness the potential of stem cells for tissue regeneration.
790
All stem cells have problems with respect to human medical applications. Adult stem cells have a limited capacity to regenerate tissues, are generally present in small numbers, and are hard to isolate from an adult human. Embryonic stem cells have much greater differentiation potential and can be cultured to generate large numbers of cells. However, their use is accompanied by ethical concerns related to the necessary destruction of human embryos. Identifying a source of plentiful and medically useful stem cells that does not raise ethical concerns remains a major goal of medical research.
Our ability to culture stem cells (i.e., maintain them in an undifferentiated state), and to manipulate them to grow and differentiate into particular tissues, is very much a function of our understanding of developmental biology. The identification and culturing of pluripotent stem cells from human blastocysts was reported by James Thomson and his colleagues in 1998. This advance led to the long-
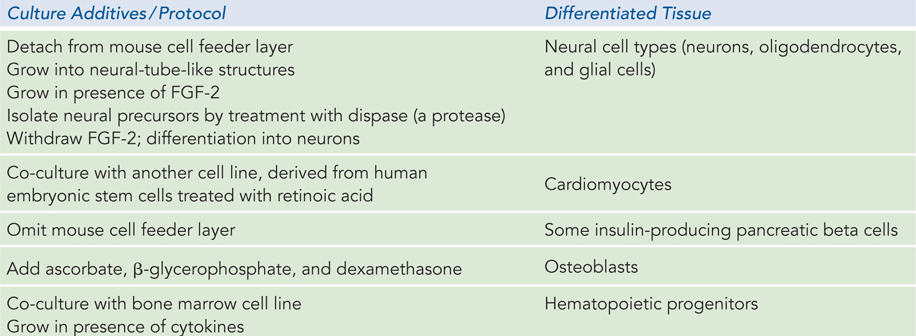
In early work, mouse and human embryonic stem cells were used for most research. Although both types of stem cells are pluripotent, they require very different culture conditions, optimized to allow cell division indefinitely without differentiation. Mouse embryonic stem cells are grown on a layer of gelatin and require the presence of leukemia inhibitory factor (LIF). Human embryonic stem cells are grown on a feeder layer of mouse embryonic fibroblasts and require basic fibroblast growth factor (bFGF or FGF-
The answer is to find another, more abundant and noncontroversial source of pluripotent stem cells. In 2007, researchers first reported success in reprogramming somatic cells to pluripotency. Skin cells—
791
SECTION 22.5 SUMMARY
Development of a multicellular organism presents the most complex regulatory challenge.
The fate of cells in the early embryo is determined in part by establishment of anterior-
posterior and dorsal- ventral gradients of proteins that act as transcription activators or translational repressors, regulating the genes required for the development of structures appropriate to a particular part of the organism. Sets of regulatory genes operate in temporal and spatial succession, transforming given areas of an egg cell into predictable structures in the adult organism.
The developmental fate of cell lineages during development is also shaped by cell-
cell signaling pathways in which signals from one cell lineage affect the fate of others. The Wnt- class signaling pathway is one well- studied example. In vertebrates, stem cells retain significant developmental potential. The differentiation of stem cells into functional tissues can be controlled by extracellular signals and conditions.