module 4 Systems and Matter
All questions about the environment involve matter, the atoms and molecules that compose materials, and the systems in which the matter circulates. In this module we will look at the basic building blocks of matter and how matter moves among systems. We will look in detail at water, an important component of most environmental systems. Finally, we will explore how matter is conserved in chemical and biological systems.
Learning Objectives
After reading this module you should be able to
describe how matter comprises atoms and molecules that move among different systems.
explain why water is an important component of most environmental systems.
discuss how matter is conserved in chemical and biological systems.
Matter comprises atoms and molecules that move among different systems
Matter Anything that occupies space and has mass.
Mass A measurement of the amount of matter an object contains.
What do rocks, water, air, the book in your hands, and the cells in your body have in common? They are all forms of matter. Matter is anything that occupies space and has mass. The mass of an object is a measurement of the amount of matter it contains. Note that the words “mass” and “weight” are often used interchangeably, but they are not the same thing. Weight is the force that results from the action of gravity on mass. Your own weight, for example, is determined by the amount of gravity pulling you toward the planet’s center. Whatever your weight on Earth, you would weigh less on the Moon where the action of gravity is weaker. In contrast, mass stays the same under any gravitational influence. So although your weight would change on the Moon, your mass would remain the same because the amount of matter you are made of would be the same. In this section we will look at important properties of matter, starting with the building blocks.
Atoms and Molecules
Atom The smallest particle that can contain the chemical properties of an element.
Element A substance composed of atoms that cannot be broken down into smaller, simpler components.
All matter is composed of tiny particles that cannot be broken down into smaller pieces. The basic building blocks of matter are known as atoms. An atom is the smallest particle that can contain the chemical properties of an element. An element is a substance composed of atoms that cannot be broken down into smaller, simpler components. At Earth’s surface temperatures, elements can occur as solids (such as gold), liquids (such as mercury), or gases (such as helium). Atoms are so small that a single human hair measures about a few hundred thousand carbon atoms across.
Periodic table A chart of all chemical elements currently known, organized by their properties.
Molecule A particle that contains more than one atom.
Compound block_type="h2"A molecule containing more than one element.
Ninety-
The Structure of an Atom
Atomic number The number of protons in the nucleus of a particular element.
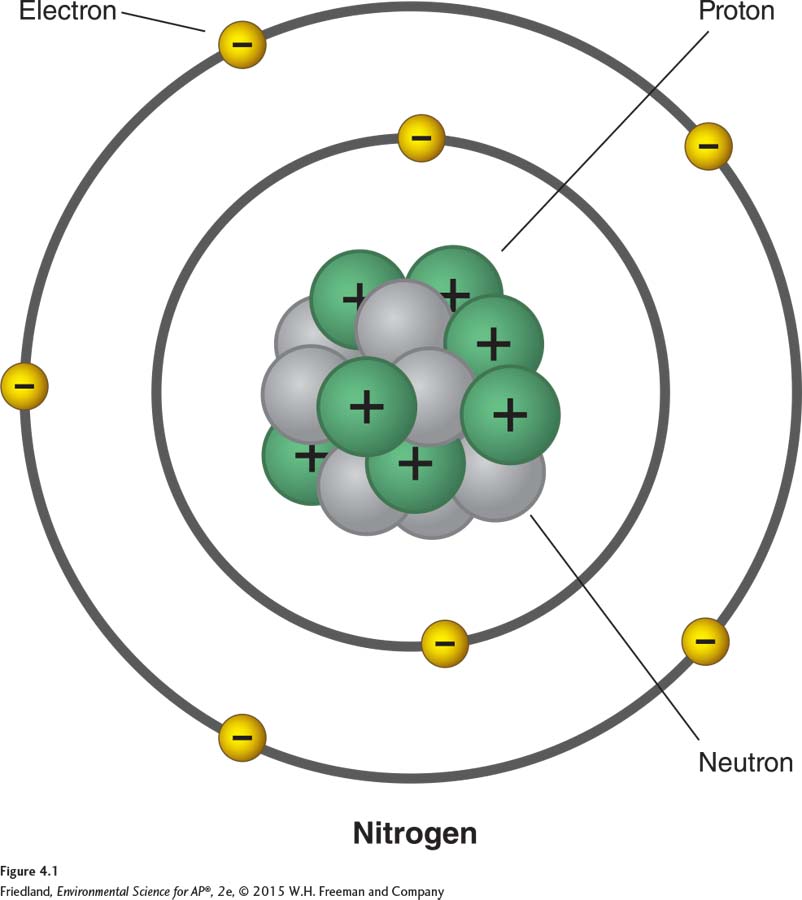
FIGURE 4.1 shows a simplified model of single atom of nitrogen. Like the nitrogen atom, every atom consists of a nucleus, or core, surrounded by electrons. The nucleus consists of protons and neutrons. Protons and neutrons have roughly the same mass—
Turning back to FIGURE 4.1 you can see that the space around the nucleus is occupied by electrons. Electrons are negatively charged, like the “minus” side of a battery, and have a much smaller mass than protons or neutrons. In the molecular world, opposites always attract, so negatively charged electrons are attracted to positively charged protons. This attraction binds the electrons to the nucleus. In a neutral atom, the numbers of protons and electrons are equal.
Mass number A measurement of the total number of protons and neutrons in an element.
The total number of protons and neutrons in an element is known as its mass number. Because the mass of an electron is insignificant compared with the mass of a proton or neutron, we do not include electrons in mass number calculations. The periodic table lists the mass number of each element under the element name.
Isotopes Atoms of the same element with different numbers of neutrons.
Although the number of protons in a chemical element is constant, atoms of the same element may have different numbers of neutrons and, therefore, different mass numbers. Atoms of the same element with different numbers of neutrons are called isotopes. Isotopes of the element carbon, for example, have six protons, but can occur with six, seven, or eight neutrons, which yield mass numbers of 12, 13, or 14, respectively. In nature, carbon occurs as a mixture of carbon isotopes. All carbon isotopes behave the same chemically. However, biological processes sometimes favor one isotope over another. Certain isotopic signatures, or ratios of isotopes, can be left behind by different biological processes. Environmental scientists use these signatures to learn about certain processes or to evaluate environmental conditions such as air pollution. Because modern carbon in wood and fossil carbon in oil or coal have different carbon isotope signatures, the proportions of different isotopes in polluted air can tell us whether the pollution comes from a forest fire or combustion of fossil fuels.
Radioactivity
Radioactive decay The spontaneous release of material from the nucleus of radioactive isotopes.
The nuclei of isotopes can be stable or unstable, depending on the mass number of the isotope and the number of neutrons it contains. Unstable isotopes are radioactive. Radioactive isotopes undergo radioactive decay, the spontaneous release of material from the nucleus. Radioactive decay changes the radioactive element into a different element. For example, uranium-
Half-
We measure radioactive decay by recording the average rate of decay of a quantity of a radioactive element. This measurement is commonly stated in terms of the half-
The measurement of isotopes has many applications in environmental science as well as in other scientific fields. For example, carbon in the atmosphere exists in a known ratio of the isotopes carbon-
Chemical Bonds
We have seen that matter is composed of atoms, which form molecules or compounds. In order to form molecules or compounds, atoms must be able to interact or join together. This happens by means of chemical bonds of various types. Chemical bonds fall into three categories: covalent bonds, ionic bonds, and hydrogen bonds.
Covalent Bonds
Covalent bond The bond formed when elements share electrons.
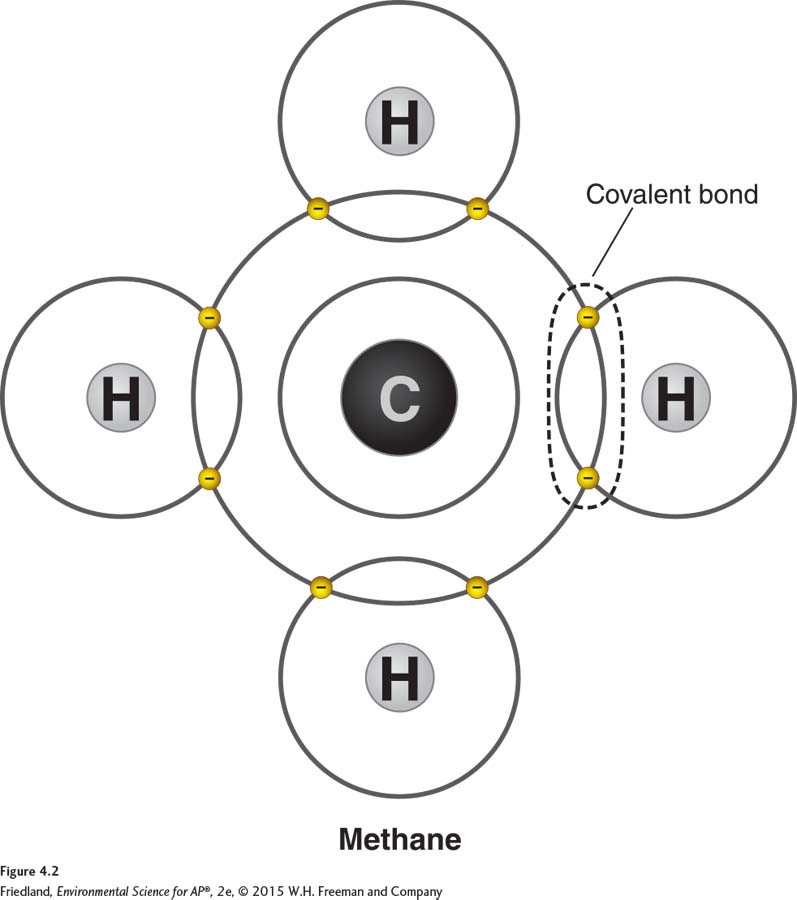
Elements that do not readily gain or lose electrons form compounds by sharing electrons. Compounds formed by sharing electrons are said to be held together by covalent bonds. FIGURE 4.2 illustrates the covalent bonds in a molecule of methane (CH4, also called natural gas). A methane molecule is made up of one carbon (C) atom surrounded by four hydrogen (H) atoms. Covalent bonds form between the single carbon atom and each hydrogen atom. Covalent bonds also hold the two hydrogen atoms and the oxygen atom in a water molecule together.
Ionic Bonds
Ionic bond A chemical bond between two ions of opposite charges.
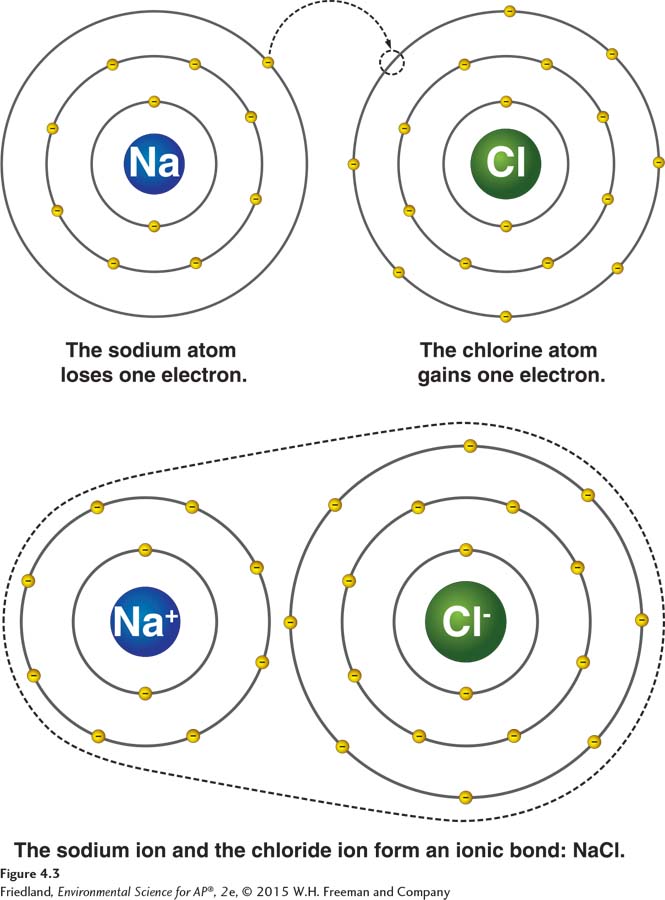
In a covalent bond, atoms share electrons. Another kind of bond between two atoms involves the transfer of electrons. When such a transfer happens, one atom becomes electron deficient (positively charged), and the other becomes electron rich (negatively charged). The charged atoms are called ions. The charge imbalance holds the two atoms together and the attraction between ions of opposite charges forms a chemical bond known as an ionic bond. FIGURE 4.3 shows an example of this process. Sodium (Na) donates one electron to chlorine (Cl), which gains one electron, to form sodium chloride (NaCl), or table salt.
An ionic bond is not usually as strong as a covalent bond. This means that the compound can readily dissolve. For example, as long as sodium chloride remains in a salt shaker, it remains in solid form. But if you shake some into water, the salt dissolves into sodium and chloride ions (Na+ and Cl−).
Hydrogen Bonds
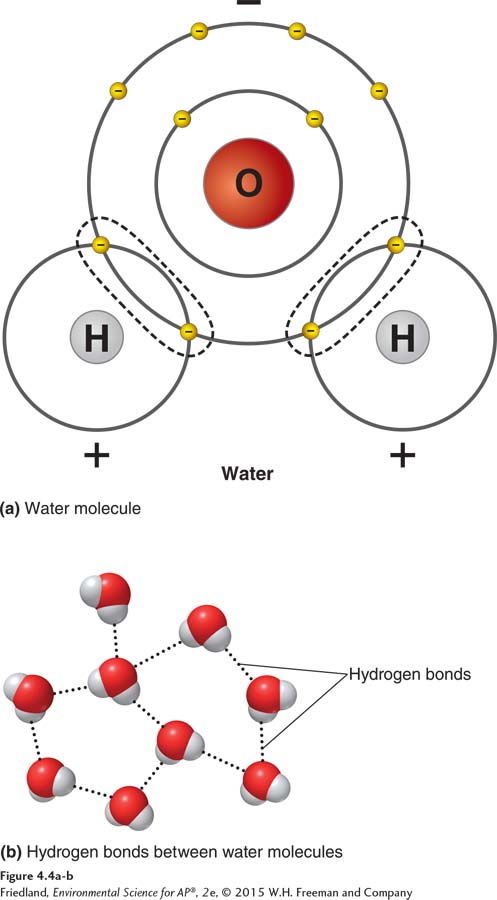
Hydrogen bond A weak chemical bond that forms when hydrogen atoms that are covalently bonded to one atom are attracted to another atom on another molecule.
The third type of chemical bond is weaker than both covalent bonds and ionic bonds. A hydrogen bond is a weak chemical bond that forms when hydrogen atoms that are covalently bonded to one atom are attracted to an atom on another molecule. When atoms of different elements form bonds, their electrons may be shared unequally; that is, shared electrons may be pulled closer to one atom than to the other. In some cases, the strong attraction of the hydrogen electron to other atoms creates a charge imbalance within the covalently bonded molecule.
Polar molecule A molecule in which one side is more positive and the other side is more negative.
Looking at FIGURE 4.4a, we see that water is an excellent example of this type of unequal electron distribution. Each water molecule as a whole is neutral; that is, it carries neither a positive nor a negative charge. But water has unequal covalent bonds between its two hydrogen atoms and one oxygen atom. Because of these unequal bonds and the angle formed by the H—
Hydrogen bonds also occur in nucleic acids such as DNA, the biological molecule that carries the genetic code for all organisms.
Water is a vital component of most environmental systems
Because water is often the vehicle for transferring chemical elements and compounds from one system to another, it is vital for environmental scientists to understand how water behaves. The molecular structure of water gives it unique properties that support the conditions necessary for life on Earth. Among these properties are surface tension, capillary action, a high boiling point, and the ability to dissolve many different substances. Each of these properties is essential to physiological functioning and the movement of elements through systems.
Surface Tension and Capillary Action
Although we don’t generally think of water as being sticky, hydrogen bonding makes water molecules stick strongly to one another in an action known as cohesion. Hydrogen bonding also makes water molecules stick strongly to certain other substances, an action known as adhesion. The ability to cohere or adhere underlies two unusual properties of water: surface tension and capillary action.
Surface Tension
Surface tension A property of water that results from the cohesion of water molecules at the surface of a body of water and that creates a sort of skin on the water’s surface.

Surface tension, which results from the cohesion of water molecules at the surface of a body of water, creates a sort of skin on the water’s surface. Have you ever seen an aquatic insect, such as a water strider, walk across the surface of the water? This is possible because of surface tension (FIGURE 4.5). Surface tension also makes water droplets smooth and more or less spherical as they cling to a water faucet before dropping.
Capillary Action
Capillary action A property of water that occurs when adhesion of water molecules to a surface is stronger than cohesion between the molecules.
Capillary action happens when adhesion of water molecules to a surface is stronger than cohesion between the molecules. The absorption of water by a paper towel or a sponge is the result of capillary action. This property is important in thin tubes, such as the water-
Boiling and Freezing
At the atmospheric pressures found at Earth’s surface, water boils (becomes a gas) at 100°C (212°F) and freezes (becomes a solid) at 0°C (32°F). If water behaved like structurally similar compounds such as hydrogen sulfide (H2S), which boils at −60°C (−76°F), it would be a gas at typical Earth temperatures and life as we know it could not exist. Because of cohesion, however, water can be a solid, a gas, or—
Water has another unique property: It takes up a larger volume in solid form than it does in liquid form. FIGURE 4.6 illustrates the difference in molecular structure between liquid water and ice. As liquid water cools, it becomes denser, until it reaches 4°C (39°F), the temperature at which it reaches maximum density. As it cools from 4°C down to freezing at 0°C, its molecules realign into a crystal lattice structure, and its volume expands. You can see the result any time you add an ice cube to a drink: Ice floats on liquid water.

What does this unique property of water mean for life on Earth? Imagine what would happen if water acted like most other liquids. As it cooled, it would continue to become denser. Its solid form (ice) would sink, and lakes and ponds would freeze from the bottom up. As a result, very few aquatic organisms would be able to survive in temperate and cold climates.
Water as a Solvent
In our table salt example, we saw that water makes a good solvent. Many substances, such as table salt, dissolve well in water because their polar molecules bond easily with other polar molecules. This explains the high concentrations of dissolved ions in seawater as well as the capacity of living organisms to store many types of molecules in solution in their cells. Unfortunately, many toxic substances also dissolve well in water, which makes them easy to transport through the environment. Fertilizers, human waste, and road deicers such as road salt are all pollutants that dissolve easily in water and so are transported far from their sources.
Acids, Bases, and pH
Acid A substance that contributes hydrogen ions to a solution.
Base A substance that contributes hydroxide ions to a solution.
Another important property of water is its ability to dissolve hydrogen or hydroxide-
When an acid is dissolved in water, it dissociates into positively charged hydrogen ions (H+) and negatively charged ions. Two important acids we will discuss in this book are nitric acid (HNO3) and sulfuric acid (H2SO4), the primary constituents of acid deposition, one form of which is acid rain.
Bases, on the other hand, dissociate into negatively charged hydroxide ions (OH−) and positively charged ions. Some examples of bases are sodium hydroxide (NaOH) and calcium hydroxide (Ca(OH)2), which can be used to neutralize acidic emissions from power plants.
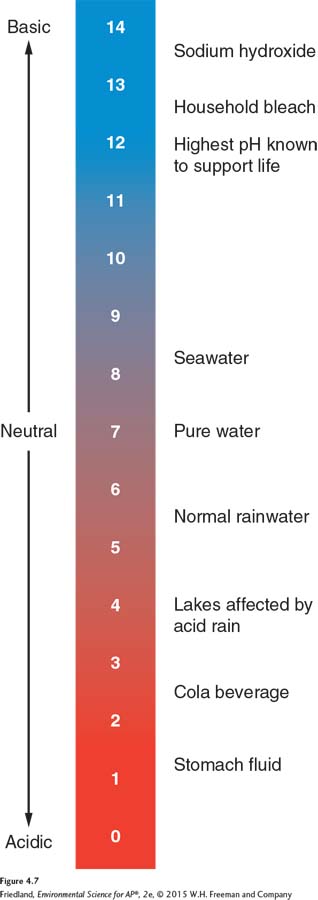
pH The number that indicates the relative strength of acids and bases in a substance.
The pH indicates the relative strength of acids and bases in a substance. A pH value of 7—
Environmental systems contain both chemical and biological reactions
The chemical principles that we have described play an important role in many environmental systems through chemical and biological reactions. Although we will look at biological and chemical reactions separately in Chapter 3, here we shall see that chemical and biological components interact in most environmental systems.
Chemical Reactions and the Conservation of Matter
Chemical reaction A reaction that occurs when atoms separate from molecules or recombine with other molecules.
A chemical reaction occurs when atoms separate from molecules or recombine with other molecules. In a chemical reaction, no atoms are ever destroyed or created, although the bonds between particular atoms may change. For example, when methane (CH4) is burned in air, it reacts with two molecules of oxygen (2 O2) to create one molecule of carbon dioxide (CO2) and two molecules of water (2 H2O):
CH4 + 2 O2 → CO2 + 2 H2O
Notice that the number of atoms of each chemical element is the same on each side of the reaction.
Chemical reactions can occur in either direction. For example, during the combustion of fuels, nitrogen gas (N2) combines with oxygen gas (O2) from the atmosphere to form two molecules of nitrogen oxide (NO), which is an air pollutant:
N2 + O2 → 2 NO
This reaction can also proceed in the opposite direction:
2 NO → N2 + O2
Law of conservation of matter A law of nature stating that matter cannot be created or destroyed; it can only change form.
The observation that no atoms are created or destroyed in a chemical reaction leads us to the law of conservation of matter, which states that matter cannot be created or destroyed; it can only change form. For example, when paper burns, it may seem to vanish, but no atoms are lost. In this case, the carbon and hydrogen that make up the paper combine with oxygen in the air to produce carbon dioxide, water vapor, and other materials, which either enter the atmosphere or form ash. Combustion converts most of the solid paper into gases, but all of the original atoms remain. The same process occurs in a forest fire, but on a much larger scale (FIGURE 4.8). The only known exception to the law of conservation of matter occurs in nuclear reactions, in which small amounts of matter change into energy.

The law of conservation of matter explains why we cannot easily dispose of hazardous materials. If something is hazardous, it typically will remain hazardous even if we try to convert it through combustion. For example, when we burn material that contains heavy metals, such as an automotive battery, the atoms of the metals in the battery do not disappear. They turn up elsewhere in the environment, where they may harm humans and other organisms. For this and other reasons, understanding the law of conservation of matter is crucial to the study of environmental science.
Biological Molecules and Cells
Inorganic compound A compound that does not contain the element carbon or contains carbon bound to elements other than hydrogen.
Organic compound A compound that contains carbon-
As we mentioned earlier, chemical and biological reactions happen simultaneously in most environmental systems. We have seen how chemical compounds form and how they respond to various processes such as burning and freezing. To understand biological processes, we must first look at the distinction between compounds that are inorganic and those that are organic. Inorganic compounds are compounds that either do not contain the element carbon or contain carbon that is bound to elements other than hydrogen. Examples include ammonia (NH3), sodium chloride (NaCl), water (H2O), and carbon dioxide (CO2). Organic compounds are compounds that have carbon-
Organic compounds are the basis of the biological molecules that are important to life: carbohydrates, proteins, nucleic acids, and lipids. Because these four types of molecules are relatively large, they are also known as macromolecules.
Carbohydrates
Carbohydrate A compound composed of carbon, hydrogen, and oxygen atoms.
Carbohydrates are compounds composed of carbon, hydrogen, and oxygen atoms. Glucose (C6H12O6) is a simple sugar (a monosaccharide, or single sugar) easily used by plants and animals for quick energy. Sugars can link together in long chains called complex carbohydrates, or polysaccharides (many sugars). For example, plants store energy as starch, which is made up of long chains of covalently bonded glucose molecules. The starch can also be used by animals that eat the plants. Cellulose, a component of plant leaves and stems, is another polysaccharide consisting of long chains of glucose molecules. Cellulose is the raw material for cellulosic ethanol, a type of fuel that has the potential to replace or supplement gasoline.
Proteins
Protein A critical component of living organisms made up of a long chain of nitrogen-
Proteins, a critical component of living organisms, are made up of long chains of nitrogen-
Nucleic Acids
Nucleic acid Organic compounds found in all living cells.
DNA (deoxyribonucleic acid) A nucleic acid, the genetic material that contains the code for reproducing the components of the next generation, and which organisms pass on to their offspring.
RNA (ribonucleic acid) A nucleic acid that translates the code stored in DNA, which makes possible the synthesis of proteins.
Nucleic acids are organic compounds found in all living cells. Long chains of nucleic acids form DNA and RNA. DNA (deoxyribonucleic acid) is the genetic material organisms pass on to their offspring; it contains the code for reproducing the components of the next generation. RNA (ribonucleic acid) translates the code stored in DNA, which makes possible the synthesis of proteins.
Lipids
Lipid A smaller organic biological molecule that does not mix with water.
Lipids are smaller biological molecules that do not mix with water. Fats, waxes, and steroids are all lipids. Lipids form a major part of the membranes that surround cells.
Cells
We have looked at the four types of macromolecules required for life. But how do they work as part of a living organism? The smallest structural and functional component of organisms is known as a cell.
Cell A highly organized living entity that consists of the four types of macromolecules and other substances in a watery solution, surrounded by a membrane.
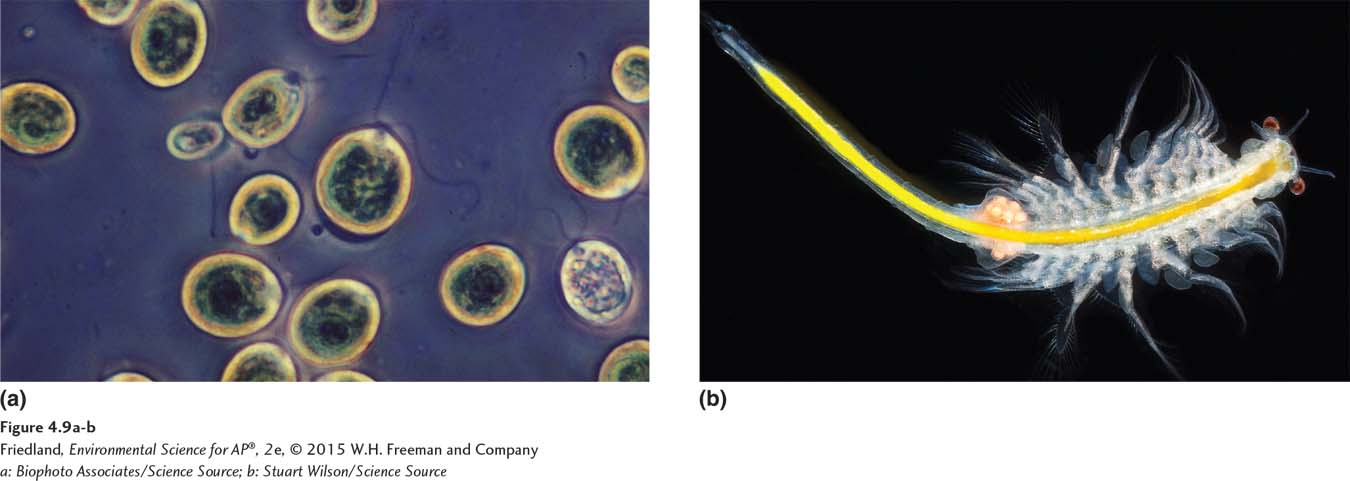
A cell is a highly organized living entity that consists of the four types of macromolecules and other substances in a watery solution, surrounded by a membrane. Some organisms, such as most bacteria and some algae, consist of a single cell. This cell contains all of the functional structures, or organelles, needed to keep the cell alive and allow it to reproduce (FIGURE 4.9a). Larger and more complex organisms, such as Mono Lake’s brine shrimp, are multicellular (FIGURE 4.9b). Throughout this book, we will be examining biological reactions and the resulting effects on the environment. Some effects are at the cellular level, while others are at the organismal level. Each is important to understanding environmental science.