1.4 Air Pollution
Identify major atmospheric pollutant types and discuss their effects on human health.
Air pollution is defined as harmful concentrations of gases or aerosols in the atmosphere. Air pollution is often called smog. This term comes from combining “smoke” and “fog,” but fog has nothing to do with air pollution. Air pollutants originate from many different sources, natural as well as anthropogenic. Wildfires, for example, produce carbon monoxide, a toxic gas, and volcanoes release sulfur compounds.
air pollution
Harmful concentrations of gases or aerosols in the atmosphere.
This section focuses on anthropogenic pollutants, the majority of which come from the burning of fossil fuels. Fossil fuels are the ancient remains of plants preserved in the lithosphere in the form of coal, oil, and natural gas. When they are burned, greenhouse gases and toxic compounds are produced and released to the atmosphere.
fossil fuels
The ancient remains of plants preserved in the lithosphere in the form of coal, oil, and natural gas.
There are two main categories of anthropogenic air pollutants: primary pollutants and secondary pollutants. A primary pollutant enters the air or water directly from its source, such as a car’s tailpipe or a factory smokestack. Examples of primary pollutants are carbon monoxide (CO), sulfur dioxide (SO2), nitrogen dioxide (NO2), and anthropogenic volatile organic compounds (VOCs). A secondary pollutant is not emitted directly from a source, but forms in the air or water through chemical reactions among primary pollutants.
primary pollutant
A pollutant that enters the air or water directly from its source.
secondary pollutant
A pollutant that is not directly emitted from a source, but forms through chemical reactions among primary pollutants in air or water.
Primary Pollutants
Carbon Monoxide
Carbon monoxide (CO) is a toxic odorless and invisible gas. When breathed in large amounts, CO reduces oxygen concentrations in the blood. Symptoms of CO poisoning include slowed reflexes and drowsiness. Death can happen within 1 to 3 minutes of exposure to high concentrations of CO gas.
carbon monoxide (CO)
A toxic odorless and invisible gas.
There are many natural sources of CO, including volcanic eruptions, forest fires, and bacterial processes as well as natural chemical reactions in the troposphere. These natural emissions far exceed anthropogenic emissions, but the natural emissions are not concentrated locally. Instead, they form low background concentrations that are mixed and dispersed in the troposphere. As a result, they present little threat to people.
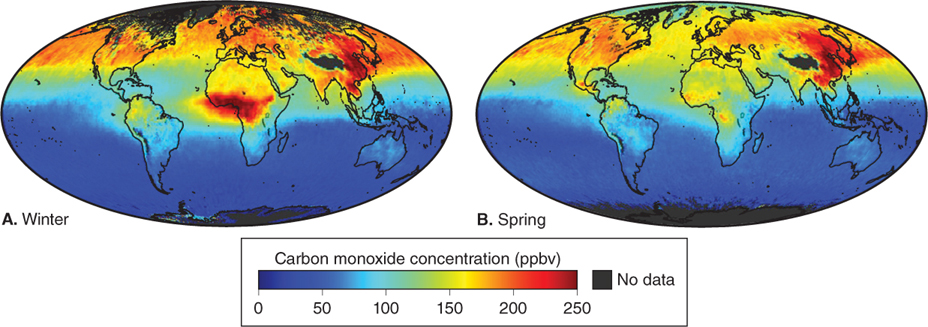
Anthropogenic carbon monoxide is formed mostly by the incomplete burning of carbon fuels (such as wood, gasoline, or coal). In urban areas in the United States, automobiles are the main source of CO emissions, producing up to 95% of the CO in the air. Clearing and burning of tropical rainforests also produces significant CO emissions in remote areas, particularly in the Southern Hemisphere, as Figure 1.10 shows.
Sulfur Dioxide
Sulfur compounds (often denoted SOx) are emitted from both natural and anthropogenic sources. Sulfur dioxide (SO2) is produced by volcanic eruptions as well as by burning of fossil fuels. In high concentrations, it is a pungent gas that causes human health problems, including irritated lung tissue and the worsening of respiratory illnesses such as bronchitis and asthma.
sulfur dioxide (SO2)
A pungent gas, produced by volcanic eruptions and by the burning of fossil fuels, that causes human health problems and acid rain.
Sulfur dioxide is the most prevalent sulfur compound produced by human activities. Over 80% of anthropogenic SO2 emissions originate from the burning of coal to generate electricity. Sulfur dioxide combined with nitrogen compounds, such as nitrogen dioxide, forms droplets of sulfuric acid (H2SO4). These droplets can then precipitate and fall to Earth as acid rain: rainfall that has a lowered pH because it has mixed with sulfur compounds. Naturally acidic rain occurs downwind of volcanic eruptions. Anthropogenic acid rain occurs mostly near industrial areas. Over time, it dissolves certain types of rock, such as limestone and marble, that are often used in building construction and statues. It also can change soil and water chemistry, causing extensive ecosystem damage (Figure 1.11).
acid rain
Rainfall that has a lowered pH because it has mixed with sulfur compounds.
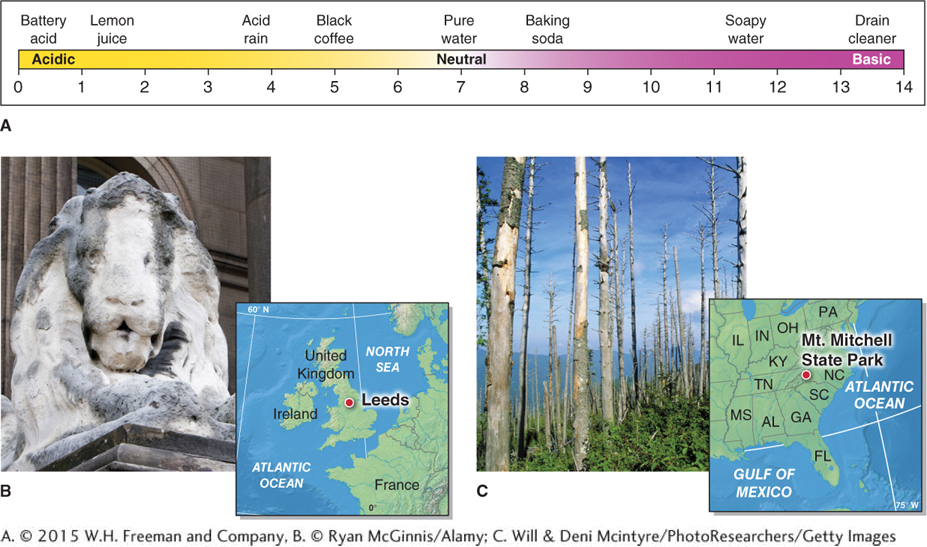
In the United States, the acid rain problem has been much improved by passage of the Clean Air Act in 1963. In recent years, life has begun returning to formerly sterile lakes due to significant improvements in air quality and the resulting decrease in acid rain. In the last decade, however, Asia’s increasing economic activity and coal burning have produced considerable acid rain problems there. Roughly a third of China has been hard hit by acid rain in the last decade, but the problem is improving. From 2006 to 2010. China’s SO2 emissions decreased by about 13% due to stronger rules governing those emissions.
Nitrogen Dioxide
In the United States, nitrogen compounds (often denoted NOx) enter the atmosphere mainly through combustion of gasoline in cars and the burning of coal to generate electricity. An important nitrogen compound is nitrogen dioxide (NO2), a toxic reddish-
nitrogen dioxide (NO2)
A toxic reddish-
Volatile Organic Compounds
Volatile organic compounds (VOCs) (also called hydrocarbons) are toxic compounds of hydrogen and carbon. Examples of VOCs include methane, butane, propane, and octane. The methane that leaks from natural gas wells, discussed in the Human Sphere section at the start of this chapter, is a VOC. This group of chemicals may irritate the eyes, nose, and throat. They can also lead to liver, kidney, and central nervous system damage. Anthropogenic emissions come mostly from industrial processes and from automobiles, when gasoline is burned incompletely and when gasoline evaporates at gas stations. Natural bacterial decomposition of organic material such as vegetation and animal waste also contributes dispersed low concentrations of methane to the atmosphere. VOCs react with other compounds in the air, creating toxic secondary pollutants.
volatile organic compound (VOC)
A toxic compound of hydrogen and carbon; also called a hydrocarbon.
Secondary Pollutants
As we have seen, some secondary pollutants are a result of chemical reactions in the atmosphere. Photochemical smog is air pollution formed by the action of sunlight on tailpipe emissions. In this section, we will cover only one component of photochemical smog: ground-
photochemical smog
Air pollution formed by the action of sunlight on tailpipe emissions.
 Ozone (O3) is a molecule that is a pollutant in the lower atmosphere. In the stratosphere, however, it blocks harmful solar UV radiation. Without stratospheric ozone, these harmful rays would be deadly to organisms living on land. This topic is explored further in the Geographic Perspectives at the end of the chapter.
Ozone (O3) is a molecule that is a pollutant in the lower atmosphere. In the stratosphere, however, it blocks harmful solar UV radiation. Without stratospheric ozone, these harmful rays would be deadly to organisms living on land. This topic is explored further in the Geographic Perspectives at the end of the chapter.
ozone (O3)
A molecule that is a pollutant in the lower atmosphere, but blocks harmful solar UV radiation in the stratosphere.
Where ozone forms near the land surface, it is called ground-
Ozone irritates the eyes and nose, scars lung tissue, and can worsen respiratory ailments such as bronchitis, emphysema, and asthma. In many urban areas, local authorities declare summer “Ozone Action Days” or “Clean Air Alerts” to warn residents to restrict their outdoor activity during high-
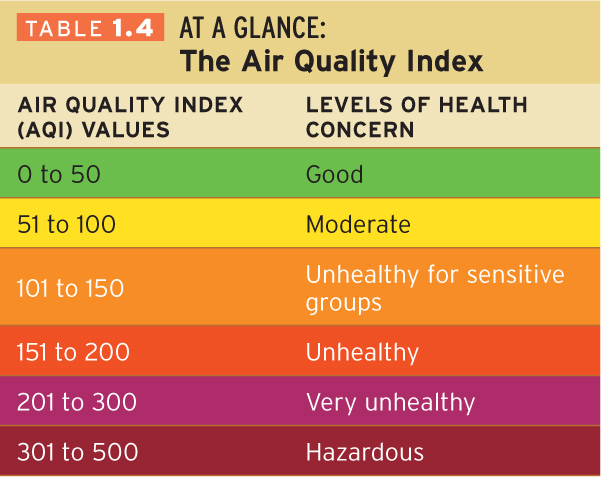
In the United States, the Environmental Protection Agency (EPA) has created the Air Quality Index (or AQI). Canada uses a similar system, called the Air Quality Health Index. The AQI system ranks daily air quality in all major metropolitan regions from 0 to 500. These rankings are based on a number of pollutants in addition to ozone. An AQI of 151 or greater is considered unhealthy for all people (Table 1.4).
Ozone corrodes rubber and plastics unless they are specially treated. Ground-
Particulate Matter
Particulate matter (PM) consists of liquid and solid particles (aerosols) suspended in the atmosphere. Particulate matter can be either a primary pollutant or a secondary pollutant, depending on whether it was directly emitted from a source or whether it resulted from a chemical reaction. Particulates can be natural or anthropogenic; they include volcanic dust and sulfuric acid droplets, pollen, fire smoke, and dust. Black carbon soot is a type of particulate matter that results from the burning of fossil fuels, vegetation, and animal dung (an important source of cooking fuel in many developing nations). Particulate matter emissions in the United States come mainly from coal burning (about 40%), industrial processes (about 23%), and transportation (about 20%).
particulate matter (PM)
Liquid and solid particles (aerosols) suspended in the atmosphere.
Particulate matter size is typically given in micrometers (μm; 1 μm = 1 millionth of a meter). The smaller the particle, the more harmful it is to human health. PM2.5 is particulate matter of 2.5 μm diameter or less. PM10 is particulate matter of 10 μm diameter. For a sense of scale, a human hair is about 70 μm in diameter. Any inhaled particles smaller than 10 μm may move deep into the lungs and then into the bloodstream, where they can cause or worsen physical ailments, including bronchitis, asthma, various cancers, and heart disease.
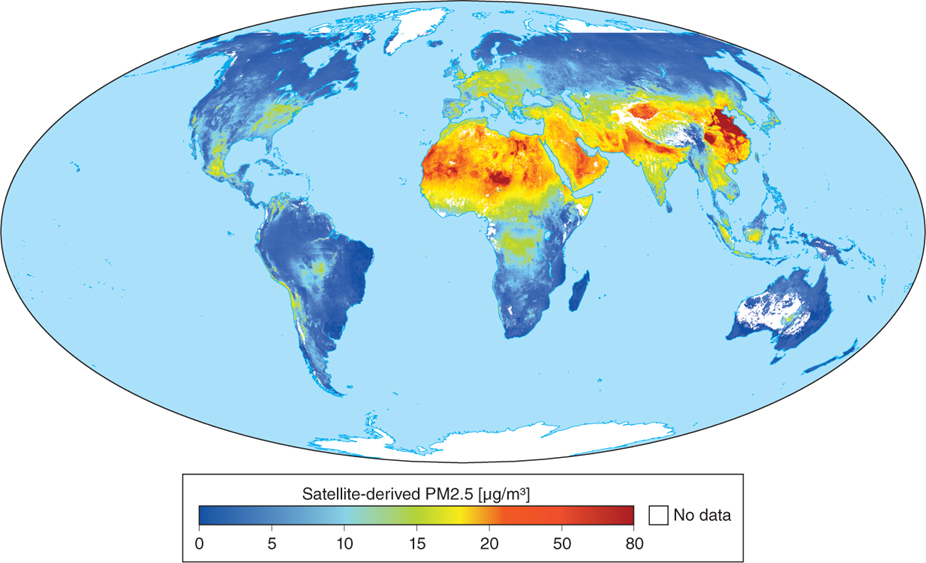
Particulate matter enters the atmosphere by means of natural dust storms in desert areas, where there is little vegetation to anchor blowing soils, and in industrial areas, as the satellite image in Figure 1.12 illustrates.
Particulate matter is removed from the atmosphere as it settles out by gravity. It is also removed as falling rain and snow carry them to Earth’s surface.
Factors That Affect Air Pollutant Concentrations
Once pollutants enter or form in the atmosphere, three factors concentrate them or carry them to other regions: topography, temperature inversions, and wind.
Topography: Valleys surrounded by mountains provide a natural basin in which air pollutants often concentrate. Many large cities are situated in flat valleys surrounded by mountains.
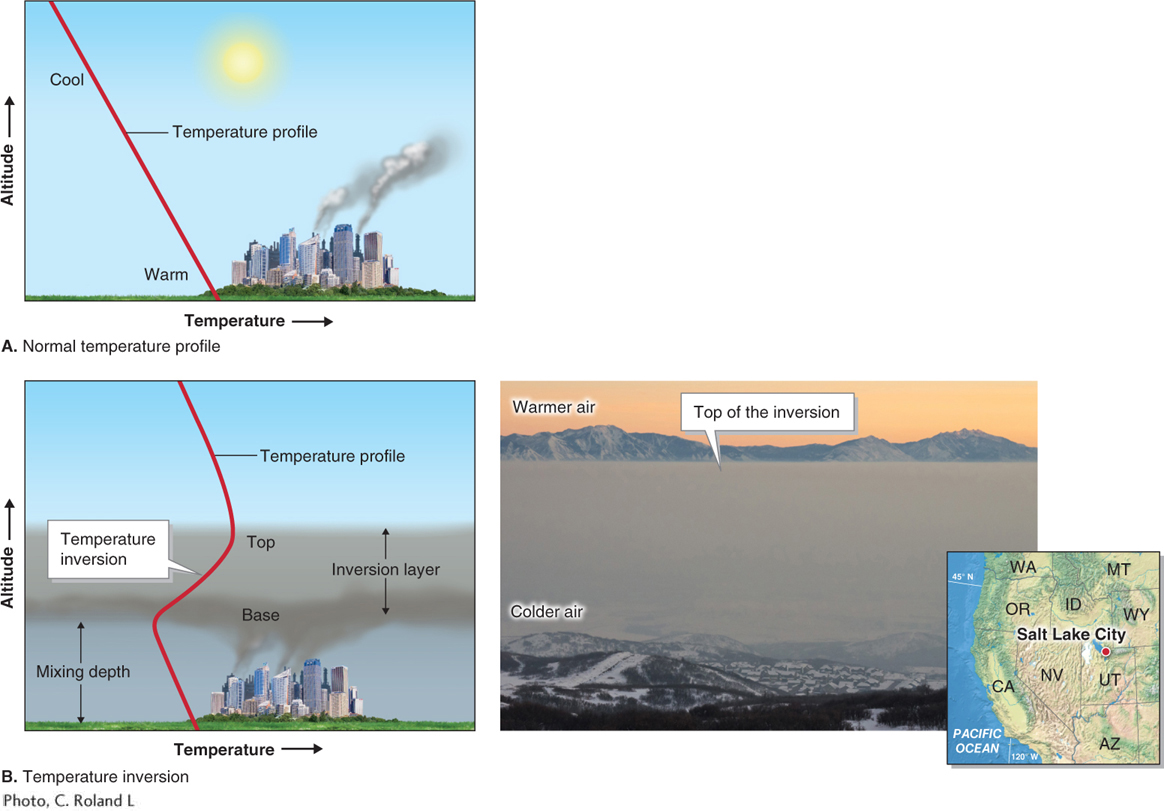
Temperature inversions: Temperature inversions often form in valleys as cold, dense air sits near the ground. These inversions can prevent air near the ground from rising higher. When pollutants are emitted into such an environment, they become concentrated near the ground. Figure 1.13 shows this process happening in Salt Lake City, Utah, where temperature inversions are particularly common.
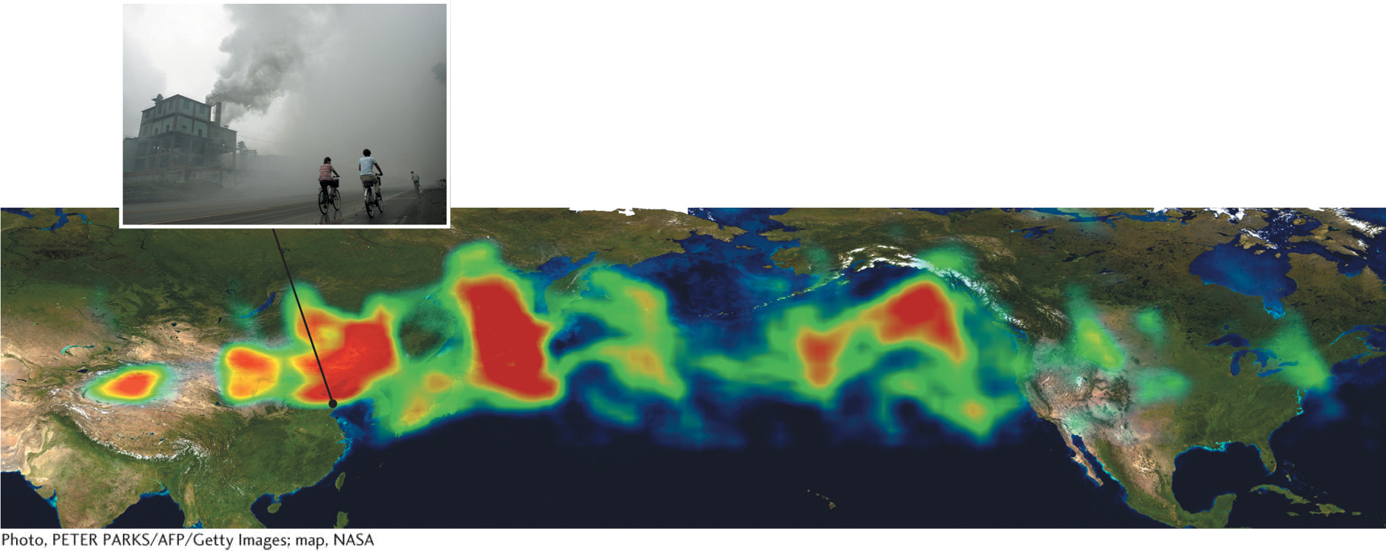
Wind: Wind typically reduces air pollution in regions where pollutants are formed or emitted by dispersing them more widely. However, wind sometimes removes pollutants from one region and concentrates them in others. North American pollution, for example, is carried by wind across the Atlantic Ocean to Europe. Similarly, particulate pollutants from industrial activity in China mix with dust storms that originate in the Gobi Desert in China and Mongolia and are then carried by wind across the Pacific to North America. The U.S. EPA has estimated that on some days, up to 25% of the particulate matter in Los Angeles originates in China. Figure 1.14 tracks a plume of pollution that moved from Asia to North America in 2001.
The Clean Air Act
Imagine that you were given the choice between fixing a minor toothache today or ignoring the problem indefinitely. You could save money by ignoring the problem and living with a little dull pain. But over time, the pain would worsen and a major dental issue would develop. Either way, the problem would have to be addressed because it would not go away, and ignoring the pain would only allow it to worsen. This is what air pollution is like. Ignoring air pollution allows it to worsen as populations grow, as people drive more cars, and as more fossil fuels are burned to meet growing energy demands.
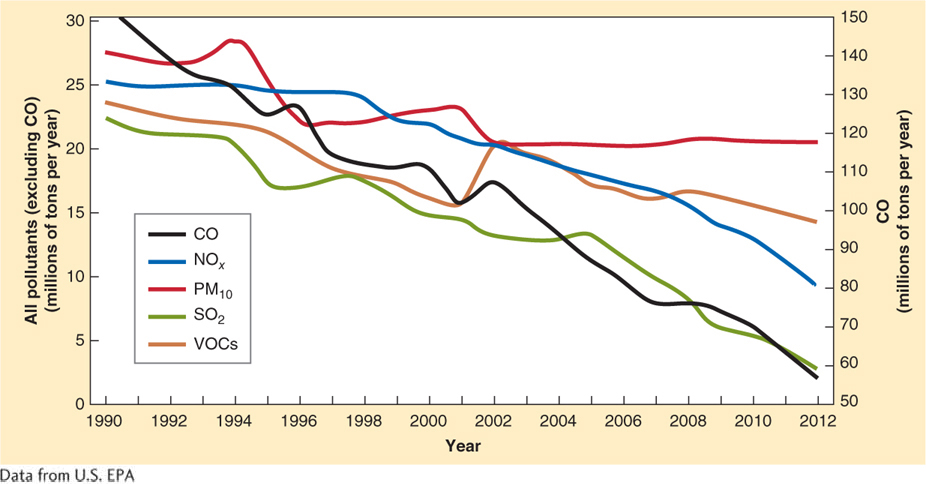
Faced with this choice, the U.S. Congress passed the Clean Air Act in 1963. It was amended in 1970, 1977, and again in 1990. The Clean Air Act imposed strict regulations on emissions of air pollutants. The Clean Air Act has been effective at reducing air pollution in the United States, saving lives, and reducing national health care–
Critics of the Clean Air Act argue that the technology necessary to reduce emissions is expensive and that implementing the law hurts the economy. They argue that enforcing clean air regulations moves jobs overseas, where it is cheaper to manufacture goods because pollution regulations are less stringent.
Proponents argue that, in the long run, clean air is cheaper than dirty air. According to the U.S. EPA, the United States will spend $65 billion to enforce the Clean Air Act for the period 1970–