1.5 Refrigerators and Life on Earth

Assess the effects of anthropogenic pollutants in the ozonosphere and the anticipated condition of the ozonosphere in the coming decades.
Before the 1930s, it was a wise practice to keep refrigerators outside and far away from the house because they occasionally blew up. Refrigerator coolants consisted of a mix of volatile and explosive chemicals, which sometimes leaked and detonated. The safety of refrigeration increased with an invention that would change the world: CFCs (chlorofluorocarbons), which are a class of ozone-
CFCs (chlorofluorocarbons)
A class of ozone-
In 1930, General Motors and Du Pont held the patent on a new chlorofluorocarbon molecule, to which they gave the trademark name Freon. CFCs were nontoxic, cheap to make, and not explosive. Within five years of their introduction, refrigerators using Freon were the standard.
CFC molecules are synthetic, meaning that they do not form naturally. Before the 1920s, there were no CFC molecules in the atmosphere. By the early 1970s, it was known that CFCs were accumulating in the atmosphere, but scientists were not much concerned because these molecules are inert, meaning that they are not chemically reactive in the lower atmosphere. Because they are inert, they have a long atmospheric lifetime. After a CFC molecule is produced, it can last in the atmosphere for 60 years, 200 years, or even more than 1,000 years, depending on the type of CFC it is. This is where the problem began.
A Landmark Paper and an International Protocol
In 1974, Mario Molina and Sherwood Rowland, both at the University of California, Irvine, published a landmark paper. They announced that CFCs linger in the troposphere for decades. Furthermore, as they mix in the atmosphere, they slowly migrate upward toward the stratosphere. (Molina and Rowland would later receive the Nobel Prize for their work.) Once in the stratosphere, CFCs are broken down by sunlight, and one of their components, chlorine, reacts with ozone, breaking it into oxygen atoms and molecules. Figure 1.16 illustrates the process of natural ozone formation and breakdown in the stratosphere as well as the role of CFCs in ozone destruction.
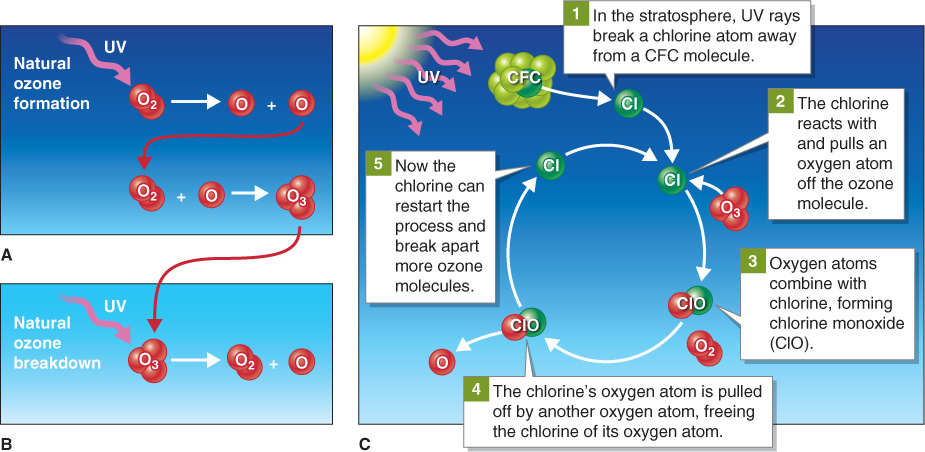
Chlorine from CFCs destroys ozone in the ozonosphere. Without the protective layer of ozone, life on land would be burned by ultraviolet rays from the Sun. Molina and Rowland recognized that in a span of only decades, CFCs had compromised Earth’s UV shield that had protected the biosphere for over 2 billion years. With that single paper published in 1974, scientists and citizens realized that there were millions of tons of CFCs in the lower troposphere working their way up to the stratosphere, breaking apart to form chlorine, and destroying ozone molecules.
Satellite and ground-
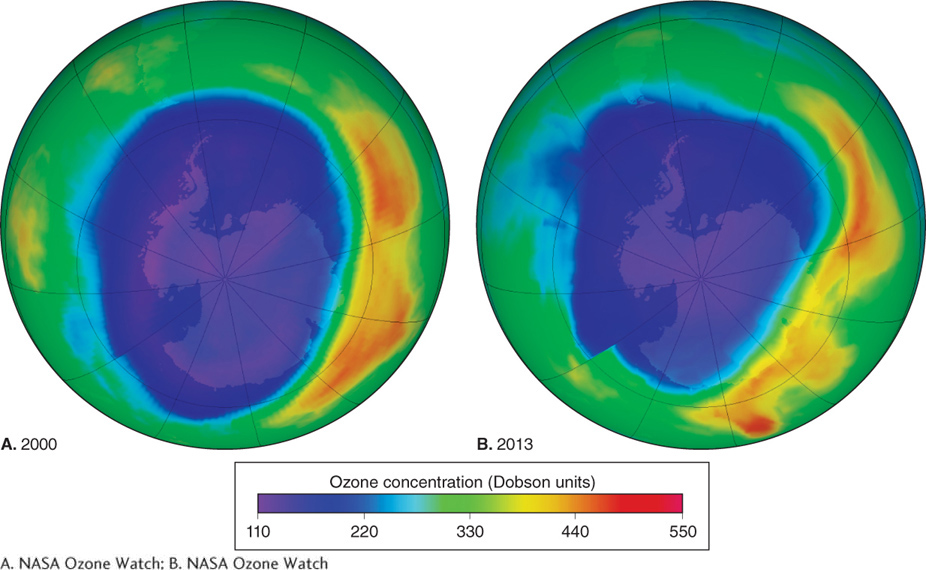
In response to this problem, the Montreal Protocol, an international agreement banning further production of CFCs, was ratified in 1987, and in 1989 it went into effect. The protocol banned CFC production outright in some countries and mandated phaseouts in other countries. Concentrations of chlorine (from CFCs) in the stratosphere over Antarctica peaked in the early 1990s and have been on the decline ever since. By about 2040, they are expected to be down to 1980 levels (Figure 1.18).
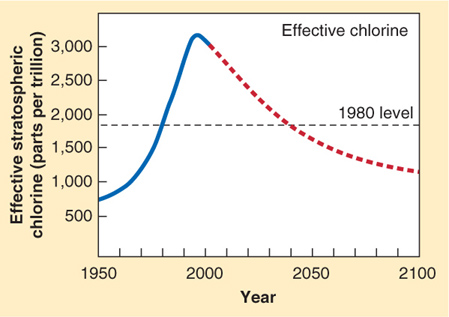
Although CFC production has been mostly phased out, the long residence time of CFCs in the troposphere means that they will continue to drift upward into the stratosphere for several more human generations. As long as there are CFCs in the atmosphere, the ozonosphere will remain vulnerable to thinning. Fortunately, stratospheric ozone is produced naturally. As the CFCs slowly disappear, the ozonosphere will repair itself.
Effects of a Thinning Ozonosphere
Without the protective ozonosphere, life on land would be impossible. Living organisms would have to retreat permanently out of the Sun. There are many problems associated with increased exposure to UV radiation.
Human Health
Skin cancer: The cause of most cases of skin cancer is UV radiation exposure. All types of skin cancer have been on the increase since the 1970s, although the increase in cases is partly a result of increased awareness and diagnosis. In the United States, melanoma (the most serious form of skin cancer) increased 800% among women and 400% among men from 1970 to 2009.
Question 1.12
Why are skin cancer rates increasing worldwide?
Increased exposure to ultraviolet rays as a result of a diminished ozonosphere has caused skin cancer rates to rise. Increased awareness and vigilance have increased reported rates of skin cancer.
Reduced immunity: The skin is an important barrier that prevents pathogens in the environment, such as harmful viruses and bacteria, from entering our bodies. When exposure to UV radiation makes our skin less healthy, these pathogens can more easily invade our bodies and make us sick.
Cataracts: Cumulative exposure to UV radiation can cause a condition called cataracts, in which the lenses of the eyes become an opaque milky white. Vision is severely impaired by cataracts.
Plants
Agriculture: Plants are stressed by, and grow more slowly when they are exposed to increased levels of UV radiation. The result is reduced agricultural production. Scientists are working to increase tolerance for UV radiation in staple crops such as rice, wheat, corn, and soybeans.
Natural ecosystems: Almost all life on Earth depends on photosynthetic organisms such as plants to convert radiant energy from the Sun to the chemical energy that fuels the biosphere. If plants grow more slowly, this chain of connectivity will be affected.
A Crisis Averted
Antarctic ozonosphere thinning peaked in 2000 and is showing a gradual trend of improvement. Had the Montreal Protocol not been enacted, and had we continued producing CFCs, the world’s ozonosphere could have disappeared by a little after the middle of the twenty-
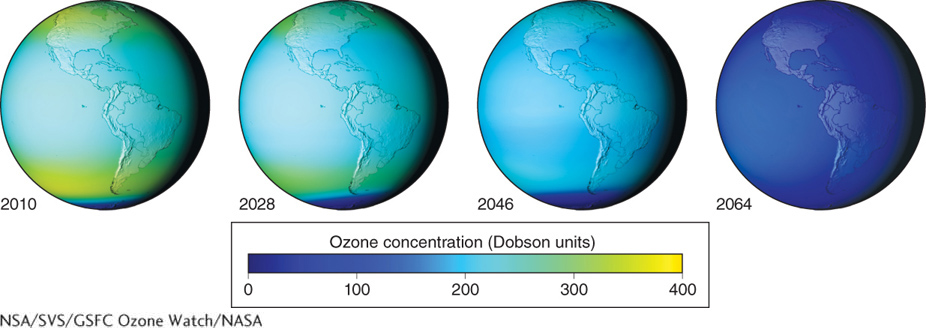
The Montreal Protocol is a good example of the international community working together to solve a problem that affects everyone. Because of the Montreal Protocol, industry has largely replaced CFCs with HFCs (hydrofluorocarbons). Unfortunately, HFCs are potent greenhouse gases: Molecule for molecule, they absorb up to twelve thousand times more energy than carbon dioxide. In a sense, we have traded the ozone-
