2.4 The Sun’s Radiant Energy
Describe solar energy and its different wavelengths.
Warm sunlight on your face is an example of the Sun’s radiant energy. Radiant energy, or radiation, is energy that is propagated in the form of electromagnetic waves, including visible light and heat. All forms of radiation have both electrical and magnetic properties, and for that reason, they are referred to as electromagnetic energy. Light is only a small portion of the electromagnetic radiation emitted by the Sun.
radiant energy
(or radiation) Energy propagated in the form of electromagnetic waves, including visible light and heat.
Electromagnetic waves travel at light speed, 300,000 km (186,000 mi) per second. The Sun is, on average, 149.6 million km (92.96 million mi) away from Earth, so sunlight takes 8.5 minutes to reach Earth. Other objects in the night sky, such as stars and galaxies, are much farther away, and the light they emitted has taken thousands or millions of years (or longer) to reach Earth.
Photons and Wavelengths
All matter emits photons (packets of energy) of electromagnetic radiation. You and this book emit photons of electromagnetic radiation, as do light bulbs, computer screens, trees, rocks, and water—
Photons travel in waves, and the distance between the peaks of two waves is the wavelength. Longer wavelengths have less energy than shorter wavelengths. The electromagnetic spectrum (EMS) is the full range of wavelengths of radiant energy, as shown in Figure 2.25. The Sun emits radiation in wavelengths across most of the EMS.
electromagnetic spectrum (EMS)
The full range of wavelengths of radiant energy.
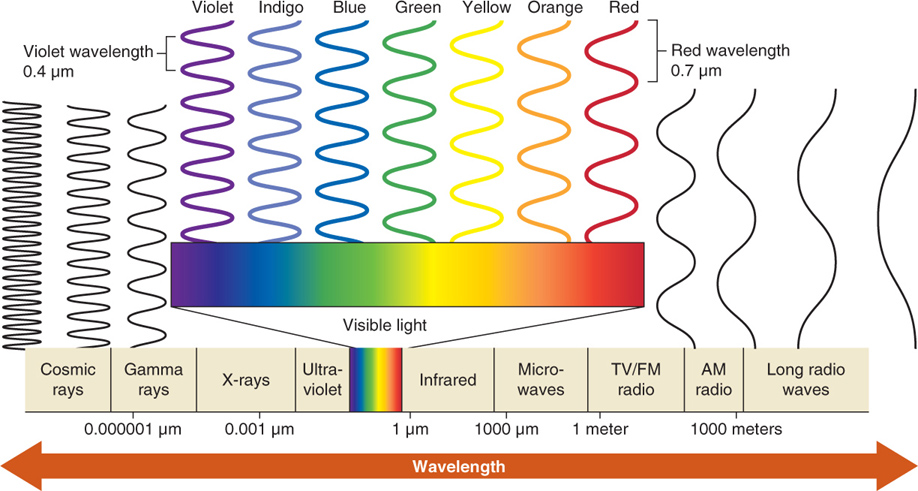
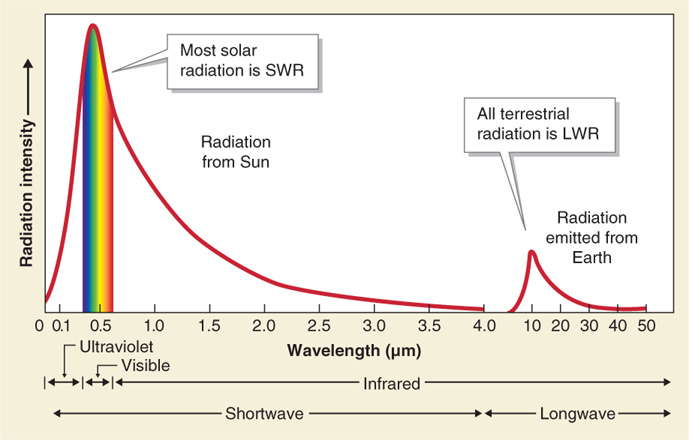
Objects with higher temperatures emit photons at shorter wavelengths than objects with lower temperatures. In addition, objects with higher temperatures emit photons at a higher rate than objects with lower temperatures. The Sun, for example, with a surface temperature of 5,800°C (10,500°F), emits energy with wavelengths centered on 0.5 μm (the visible portion of the EMS). Earth’s lower atmosphere and surface, with an average temperature of 14.6°C (58.3°F), are far cooler than the Sun and emit electromagnetic radiation with wavelengths centered on 10 μm (the infrared portion of the EMS). Being cooler, Earth also emits energy at a lower rate than the Sun.
All radiation emitted by Earth (called terrestrial radiation) is longwave radiation (LWR). LWR is defined as having wavelengths greater than 4 μm. Most solar radiation is shortwave radiation (SWR). SWR has wavelengths less than 4 μm (Figure 2.26).
longwave radiation (LWR)
Radiation with wavelengths longer than 4 μm.
shortwave radiation (SWR)
Solar radiation with wavelengths shorter than 4 μm; includes visible sunlight.
Earth’s Important Wavelengths: Ultraviolet, Visible, and Infrared
Ninety-
Ultraviolet Radiation
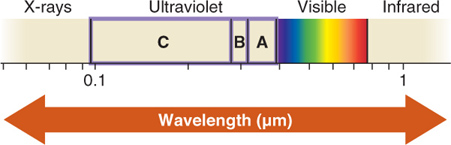
As we learned in Section 1.5, most of the Sun’s ultraviolet (UV) radiation is absorbed by ozone molecules high in the stratosphere. Clouds and aerosols also play important roles in determining how much UV radiation reaches Earth’s surface. Thick clouds can absorb almost all UV radiation, and other atmospheric aerosols also scatter and absorb it. Generally, higher elevations receive more UV radiation because there are few molecules to scatter UV rays, resulting in more intense UV exposure. In mountains, UV radiation exposure is further increased when a cover of bright snow blankets the ground. Clean snow can reflect up to 95% of UV radiation.
UV radiation is subdivided into three categories depending on its wavelength: UV-
Visible Radiation: Light
Visible radiation (or light) has wavelengths between 0.40 and 0.75 μm (see Figure 2.25). Visible radiation is the portion of the electromagnetic spectrum that we can see. Forty-
visible radiation
(or light) The portion of the electromagnetic spectrum that people can see.
Infrared Radiation
Infrared radiation (IR) has wavelengths longer than visible radiation; a portion of this radiation is heat. Infrared wavelengths range between 0.75 and 1,000 μm. Earth absorbs shortwave solar radiation and re-
infrared radiation (IR)
Electromagnetic radiation that has wavelengths longer than visible radiation.