CONCEPT39.3 The Adaptive Immune Response Is Specific
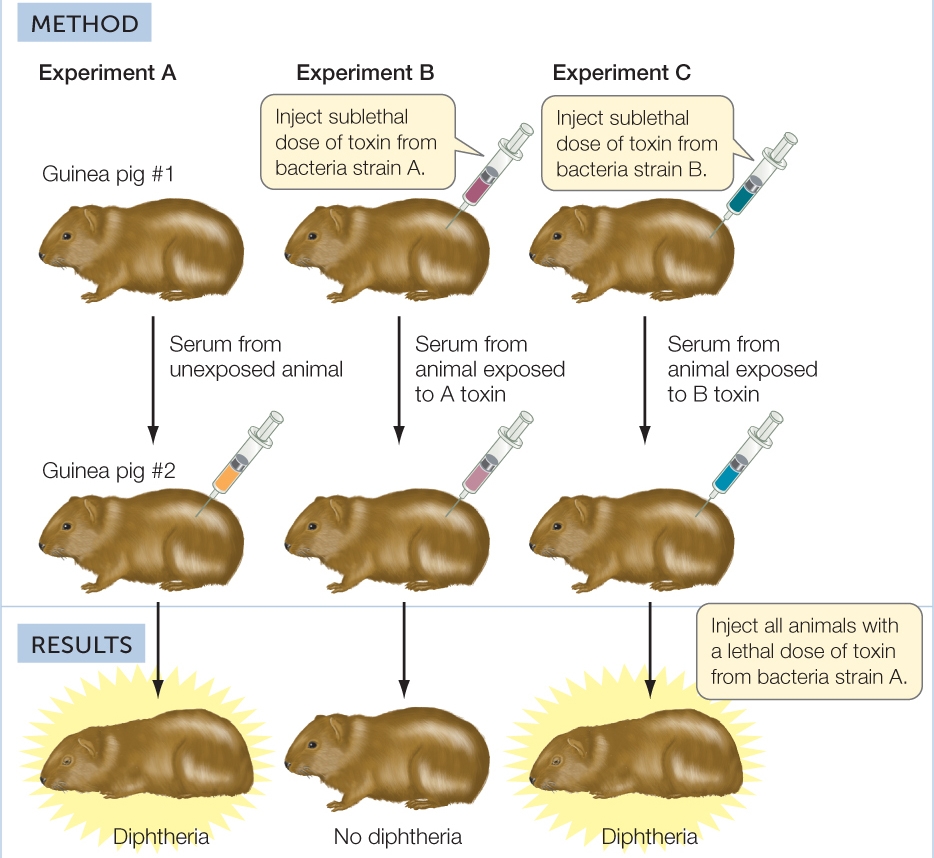
Long before the twentieth century, scientists suspected that blood was somehow involved in immunity against pathogens, but they did not have definitive experimental evidence for this. More than a century ago, Emil von Behring and Shibasaburo Kitasato at the University of Marburg in Germany performed a key experiment that suggested that blood contained important factors for adaptive immunity (FIGURE 39.5). They showed that guinea pigs injected with a sublethal dose of diphtheria toxin developed in their blood serum (the noncellular fluid that remains after blood is clotted) a factor that, when injected into other guinea pigs, was able to protect them from a lethal dose of the same toxin. In other words, the serum recipients had somehow acquired immunity. The response of the donor guinea pigs to the diphtheria toxin is an example of adaptive immunity: after exposure to the toxin, they made a protective factor that was not present before their exposure. Moreover, the immunity was specific: the factor made by the first guinea pig protected the others only against the specific toxin produced by the strain of bacteria with which the first guinea pig had been injected.
Investigation
HYPOTHESIS
Serum from guinea pigs injected with a sublethal dose of diphtheria toxin protects other guinea pigs that are exposed to a lethal dose of the same toxin.
CONCLUSION
Serum of toxin-exposed guinea pigs is protective against later exposure to a lethal dose of toxin from the same genetic strain of bacteria, but not a different strain.
ANALYZE THE DATA
In their experiments, Behring and Kitasato used different doses of toxin to test the immunity of guinea pig #2 in each experiment. The results are shown in the table.
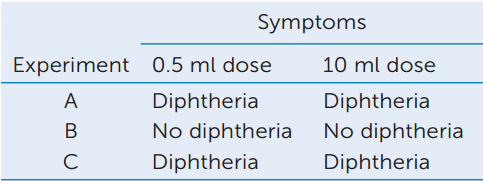
- Explain these data in terms of the level of protection afforded by the serum.
- These experiments could be performed with either intact bacteria that cause diphtheria, or with a bacteria-free filtrate of a 10-day-old culture of the bacteria. Explain.
aE. von Behring and S. Kitasato. 1890. Deutsche medizinisch Wochenschrift 16: 1113–1114.
Based on the animal model, Behring realized that serum protection might work for human diseases as well. It did, and he won the Nobel Prize for his efforts in protecting children against diphtheria. Later, the agent of this immunity was identified as an antibody protein, and the process of acquiring immunity from antibodies received from another individual was called passive immunity.
LINK
Many bacteria produce toxins of various types; see Concept 19.3
The injection of antibodies to produce a passive immune response is an artificial system that allows an organism to mount a defense against a single pathogenic strain. Vertebrates have evolved systems of adaptive immunity that have the potential to recognize and eliminate virtually any pathogen that may infect the body. We will now turn to some key features of adaptive immunity.
Adaptive immunity has four key features
Four important features of the adaptive immune system are: specificity; the ability to distinguish self from nonself; the ability to respond to an enormous diversity of nonself molecules; and immunological memory.
Specificity
B and T lymphocytes are crucial for the specificity of adaptive immune responses. T cell receptors and the antibodies produced by B cells recognize and bind to specific nonself substances called antigens, and this interaction initiates a specific immune response. Each T cell and each anti-body-producing B cell is specific for a single antigen.
The sites on antigens that the immune system recognizes are called antigenic determinants or epitopes:
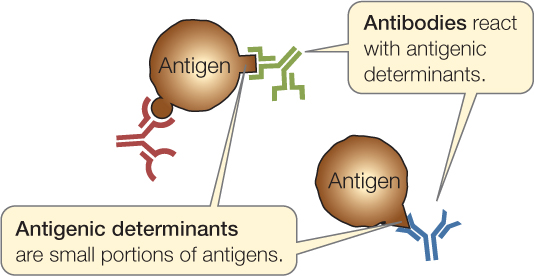
Antigens are usually proteins or polysaccharides, and there can be multiple antigens on a single invading bacterium. An antigenic determinant is a specific portion of an antigen, such as a certain sequence of amino acids that may be present in a protein. A single antigenic molecule can have multiple different antigenic determinants. For the remainder of the chapter, we will often refer to antigenic determinants simply as “antigens.”
Distinguishing Self From Nonself
As we saw in Concept 39.2, the innate immune system can recognize certain nonself molecules (PAMPs), and mount defensive responses to the pathogens from which these nonself molecules were derived. The adaptive immune system recognizes both self and non-self molecules. The human body contains tens of thousands of different molecules, each with a specific three-dimensional structure capable of generating an immune response. Thus every cell in the body bears a tremendous number of antigens. A crucial requirement of an individual’s immune system is that it recognize the body’s own antigens and not attack them.
Normally, the body is tolerant of its own molecules—the same molecules that would generate an immune response in another individual. This occurs primarily during the early differentiation of T and B cells, when they first encounter self antigens. Any immature B or T cell that shows the potential to mount a strong immune response against self antigens undergoes apoptosis within a short time. This process is called clonal deletion.
A failure of clonal deletion can lead to an immune response within an individual to self antigens, or autoimmunity. Autoimmunity does not always result in disease, but a number of autoimmune diseases are common:
- People with systemic lupus erythematosis (SLE) have antibodies to many cellular components. These antibodies can cause serious damage when they bind to normal tissue antigens and form large circulating antigen–antibody complexes. These can become stuck in tissues and provoke inflammation.
- Hashimoto’s thyroiditis is the most common autoimmune disease in women over age 50. Immune cells attack thyroid tissue, resulting in fatigue, depression, weight gain, and other symptoms associated with impaired thyroid function.
Diversity
Pathogens take many forms: viruses, bacteria, protists, fungi, and multicellular parasites. Furthermore, each pathogenic species usually exists as many subtly different genetic strains, and each strain possesses multiple surface features. Estimates vary, but a reasonable guess is that humans can respond specifically to 10 million different antigens. Upon recognizing an antigen, the immune system responds by activating lymphocytes of the appropriate specificity.
To have the ability to respond to the large number of potential pathogens, the body needs to generate a vast diversity of lymphocytes that are specific for different antigens. These lymphocytes represent a pool from which specific cells are selected when needed.
- Diversity is generated primarily by DNA changes—chromosomal rearrangements and other mutations—that occur just after the B and T cells are formed in the bone marrow. Each B cell is able to produce only one kind of antibody; thus there are millions of different B cells. Similarly, there are millions of different T cells with specific T cell receptors. The adaptive immune system is “predeveloped”—all of the machinery available to respond to an immense diversity of antigens is already there, even before the antigens are encountered.
- Antigen binding “selects” a particular B or T cell for proliferation. For example, when an antigen fits the surface receptor on a B cell and binds to it, that B cell is activated. It divides to form a clone of cells (a genetically identical group derived from a single cell), all of which produce and/or secrete antibodies with the same specificity as the receptor (FIGURE 39.6). Binding, activation, and proliferation also apply to T lymphocytes. A particular lymphocyte is selected via binding and activation, and then it proliferates to generate a clone—hence the name clonal selection for this mechanism of producing an immune response.
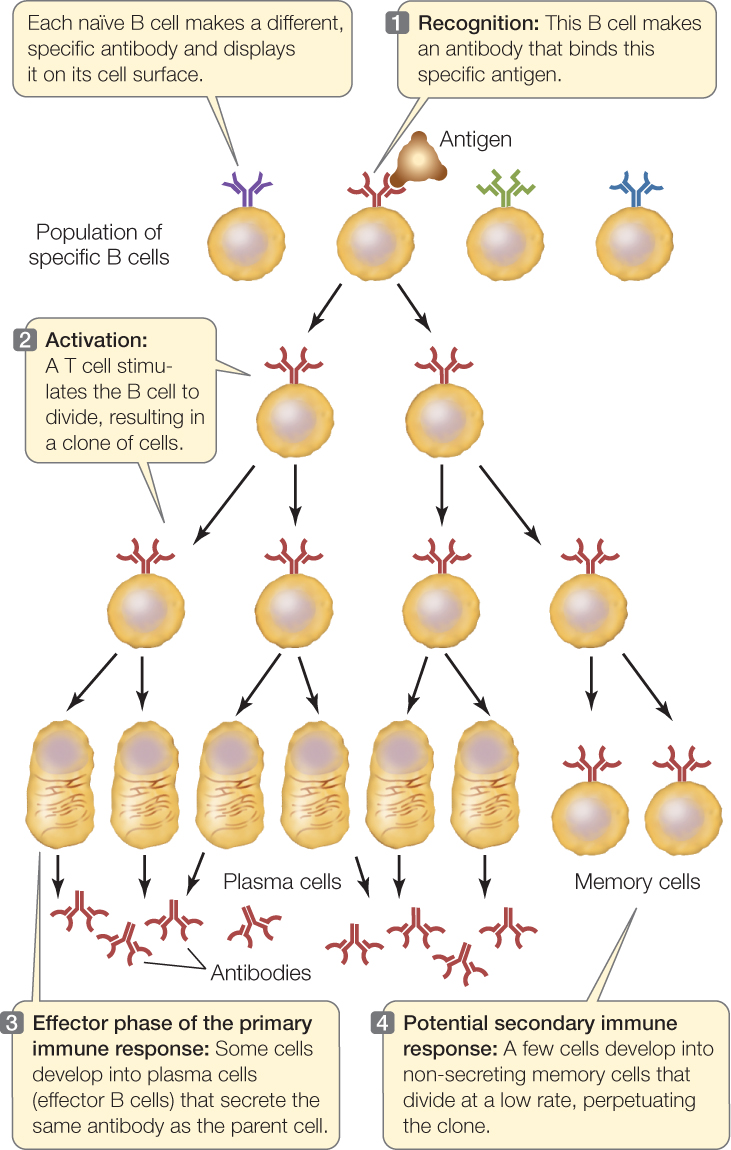 Figure 39.6: Clonal Selection in B Cells The binding of an antigen to a specific receptor on the surface of a B cell stimulates that cell to divide, producing a clone of genetically identical cells to fight that invader.
Figure 39.6: Clonal Selection in B Cells The binding of an antigen to a specific receptor on the surface of a B cell stimulates that cell to divide, producing a clone of genetically identical cells to fight that invader.
Immunological Memory
After responding to a particular type of pathogen once, the immune system “remembers” that pathogen and can usually respond more rapidly and powerfully to the same threat in the future. This immunological memory usually saves us from repeats of childhood infectious diseases.
The first time a vertebrate animal is exposed to a particular antigen, it takes several days before the adaptive immune system produces antibodies and T cells specific for the antigen. This is the primary immune response. Immunological memory arises from the fact that activated lymphocytes divide and differentiate to produce two types of daughter cells: effector cells and memory cells (see Figure 39.6).
- Effector cells carry out the attack on the antigen. Effector B cells, called plasma cells, secrete antibodies. Effector T cells release cytokines and other molecules that initiate reactions that destroy nonself or altered cells. Effector cells live for only a few days.
- Memory cells are long-lived cells that retain the ability to start dividing on short notice to produce more effector and more memory cells. Memory B and T cells may survive in the body for decades, rarely dividing.
After a primary immune response to a particular antigen, subsequent encounters with the same antigen will trigger a much more rapid and powerful secondary immune response. The memory cells that bind with that antigen proliferate, launching a huge army of plasma cells and effector T cells. The principle behind vaccination is to trigger a primary immune response that prepares the body to mount a stronger, quicker secondary response if it encounters the actual pathogen.
Macrophages and dendritic cells play a key role in activating the adaptive immune system
We have just seen how the adaptive immune system uses antigens to distinguish between self and nonself, and how it uses clonal selection to produce large numbers of specific B and T cells. In Concepts 39.4 and 39.5 we will focus on how these cells function to eliminate specific pathogens from the body. But how is the adaptive immune system activated in the first place?
One mechanism for eliminating pathogens in the innate immune system is phagocytosis. After ingestion of a pathogenic organism or infected host cell, phagocytic cells display fragments of the pathogen on their cell surfaces. These fragments function as antigens, and antigen presentation is one way that components of the innate immune system communicate with the adaptive immune system. Macrophages and dendritic cells play a key role in activating the adaptive immune system. After engulfing pathogens or infected host cells, these cells migrate to lymph nodes, where they present antigen to immature T cells. In addition, the antigen-presenting cells secrete cytokines and other signals that stimulate the activation and differentiation of the T cells.
Two types of adaptive immune responses interact
The adaptive immune system mounts two types of responses against invaders: the humoral immune response and the cellular immune response. These two responses work simultaneously and cooperatively, sharing many mechanisms. We will use the example of a viral infection in an overview of these two types of responses (FIGURE 39.7). These responses also occur when bacteria or other pathogens infect and grow inside host cells.
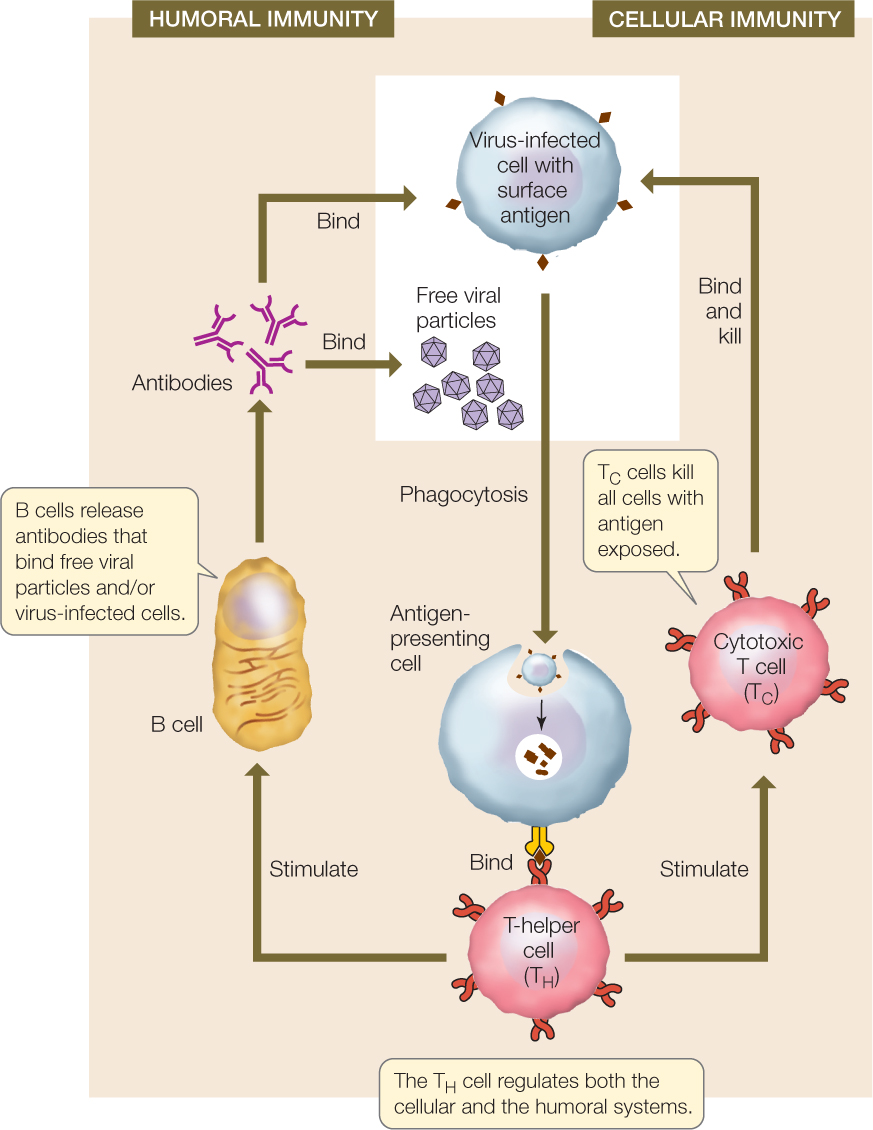
B cells that make antibodies are the workhorses of the humoral immune response, and cytotoxic T (TC) cells are the workhorses of the cellular immune response. There are three phases in both types of immune response:
- Recognition phase: In both cellular and humoral immunity, recognition occurs when an antigen is inserted into the cell membrane of an antigen-presenting cell, with the unique antigen structure protruding from the cell membrane. In addition to dendritic cells and macrophages, developing B cells can also perform phagocytosis and function as antigen-presenting cells. Figure 39.7 shows a virus-infected host cell being engulfed by an antigen-presenting cell; in other cases, the free viral particles may be engulfed. The antigen on the surface of the antigen-presenting cell is recognized by a T-helper (TH) cell bearing a T cell receptor protein that is specific for the antigen. In both humoral and cellular immunity, binding initiates the activation phase.
- Activation phase: When the TH cell recognizes an antigen on an antigen-presenting cell, it propagates and releases cytokines that stimulate B cells and TC cells bearing receptors to the same antigen to divide. The results are a clone of B cells that function in humoral immunity and a clone of TC cells that function in cellular immunity.
- Effector phase: In the humoral immune response, cells of the B clone produce antibodies that bind to viral particles and/or virus-infected cells. The bound antibodies attract phagocytes and complement proteins that engulf and destroy the virus and the virus-infected cells. In cellular immunity, cells of the TC clone bind to virus-infected cells and destroy them.
CHECKpointCONCEPT39.3
- List the four key features of the adaptive immune system.
- In 2009, the H1N1 strain of influenza was a worldwide epidemic. Notably, very old people, who had been alive during the 1918 flu outbreak, had low rates of H1N1 infection. Explain this in terms of immunological memory.
- Insulin-dependent (type 1) diabetes results from a destruction of the cells in the pancreas that make the hormone insulin (see Concept 30.5). One hypothesis for this disease is that it is caused by an autoimmune reaction. Explain how this might happen and how you would investigate your hypothesis.
The adaptive immune response is specific
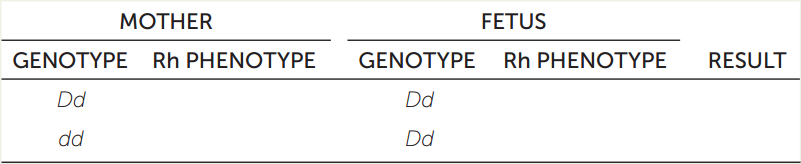
In humans, the dominant gene D codes for the RhD (Rhesus) protein on the surfaces of red blood cells. The recessive allele d does not encode a cell surface protein. If cells from an individual who is DD or Dd (Rh+) enter the bloodstream of an individual who is dd (Rh−), the Rh− individual makes antibodies that react with the Rh+ cells. During development, the blood vessels of a mother and her fetus are near each other but do not mix. However, antibodies can pass through capillary walls between the two blood supplies.
- Hemolytic disease of the newborn (sometimes resulting in stillbirth) can occur when antibodies from the mother pass into the fetal circulatory system. Fill in the table, which predicts RhD incompatibility.
- During birth there is mixing of the blood supplies of mother and fetus. In a situation of RhD incompatibility, the first birth does not result in a clinical problem. But a second RhD incompatible birth with this mother results in a hemolytic disease. Explain how this can happen (hint: immunological memory).
- Today, RhD incompatibility does not result in stillbirths. The mother is treated with serum containing anti-RhD antibodies late in the first and subsequent pregnancies. How can this prevent the immunological reactions?
With this overview of the basic characteristics of adaptive immunity and the events of the immune response, we will now turn to some of the cellular and molecular details of how these events occur.