Polydactyly
A fairly common syndrome in humans is the development of extra partial or complete digits on the hands and feet. This condition, called polydactyly, arises in about 5 to 17 of every 10,000 live births. In the most dramatic cases, the condition is present on both hands and feet (Figure 13-27). Polydactyly occurs widely throughout vertebrates—in cats, chickens, mice, and other species.
Figure 13-27: Polydactyly in humans
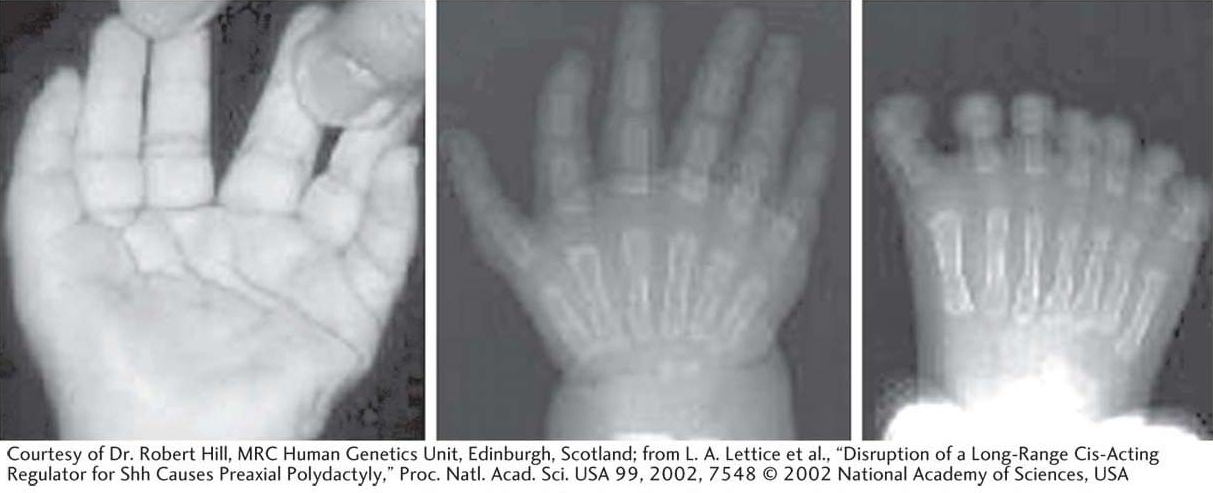
Figure 13-27: This person has six fingers on each hand and seven toes on each foot owing to a regulatory mutation in the Sonic hedgehog gene.
[Courtesy of Dr. Robert Hill, MRC Human Genetics Unit, Edinburgh, Scotland; from L. A. Lettice et al., “Disruption of a Long-Range Cis-Acting Regulator for Shh Causes Preaxial Polydactyly,” Proc. Natl. Acad. Sci. USA 99, 2002, 7548 © 2002 National Academy of Sciences, USA.]
The discovery of the role of Shh in digit patterning led geneticists to investigate whether the Shh gene was altered in polydactylous humans and other species. In fact, certain polydactyly mutations are mutations of the Shh gene. Importantly, the mutations are not in the coding region of the Shh gene; rather, they lie in a cis-acting regulatory element, far from the coding region, that controls Shh expression in the developing limb bud. The extra digits are induced by the expression of Shh in a part of the limb where the gene is not normally expressed. Mutations in cis-acting regulatory elements have two important properties that are distinct from mutations in coding regions. First, because they affect regulation in cis, the phenotypes are often dominant. Second, because only one of several cis-acting regulatory elements may be affected, other gene functions may be completely normal. Polydactyly can occur without any collateral developmental problems. Coding mutations in Shh, however, tell a different story, as we will see in the next section.
Page 502
Holoprosencephaly
Mutations in the human Shh coding region also have been identified. The consequent alterations in the Shh protein are associated with a syndrome termed holoprosencephaly, in which abnormalities occur in brain size, in the formation of the nose, and in other midline structures. These abnormalities appear to be less severe counterparts of the developmental defects observed in homozygous Shh mutant mice. Indeed, the affected children seen in clinics are heterozygous. One copy of a normal Shh gene appears to be insufficient for normal midline development (the gene is haploinsufficient). Human fetuses homozygous for loss-of-function Shh mutations very likely die in gestation with more severe defects.
Holoprosencephaly is not caused exclusively by Shh mutations. Shh is a ligand in a signal-transduction pathway. As might be expected, mutations in genes encoding other components of the pathway affect the efficiency of Shh signaling and are also associated with holoprosencephaly. Several components of the human Shh pathway were first identified as homologs of members of the fly pathway, demonstrating once again both the conservation of the genetic toolkit and the power of model systems for biomedical discovery.
Cancer as a developmental disease
In long-lived animals, such as ourselves and other mammals, development does not cease at birth or at the end of adolescence. Tissues and various cell types are constantly being replenished. The maintenance of many organ functions depends on the controlled growth and differentiation of cells that replace those that are sloughed off or otherwise die. Tissue and organ maintenance is generally controlled by signaling pathways. Inherited or spontaneous mutations in genes encoding components of these pathways can disrupt tissue organization and contribute to the loss of control of cell proliferation. Because unchecked cell proliferation is a characteristic of cancer, the formation of cancers may be a consequence. Cancer, then, is a developmental disease, a product of normal developmental processes gone awry.
Some of the genes associated with types of human cancers are shared members of the animal toolkit. For example, the patched gene encodes a receptor for the Hedgehog signaling proteins. In addition to causing inherited developmental disorders such as polydactyly and holoprosencephaly, mutations in the human patched gene are associated with the formation of a variety of cancers. About 30 to 40 percent of patients with a dominant genetic disorder called basal cell nevus syndrome (BCNS) carry patched mutations. These persons are strongly disposed to develop a type of skin cancer called basal-cell carcinoma. They also have a greatly increased incidence of medulloblastoma, a very deadly form of brain tumor. A growing list of cancers are now associated with disruptions of signal-transduction pathways—pathways that were first elucidated by these early systematic genetic screens for patterning mutants in fruit flies (Table 13-2).
Table 13-2 Some Toolkit Genes Having Roles in Cancer
|
|
|
|
Signaling-Pathway Components |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Basal-cell-carcinoma, medulloblastoma |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Acute pre-B-cell leukemia |
The discoveries of links between mutations of signal-transduction-pathway genes and human cancer have greatly facilitated the study of the biology of cancer and the development of new therapies. For example, about 30 percent of mice heterozygous for a targeted mutation in the patched gene develop medulloblastoma. These mice therefore serve as an excellent model for the biology of human disease and a testing platform for therapy. Many of the newest anticancer agents employed today are in fact targeted toward components of signal-transduction pathways that are disrupted in certain types of tumors.
Page 503
It is fair to say that even the most optimistic and farsighted researchers did not expect that the discovery of the genetic toolkit for building a fly would have such far-ranging effects on understanding human development and disease. But such huge unforeseen dividends are familiar in the recent history of basic genetic research. The advent of genetically engineered medicines, monoclonal antibodies for diagnosis and therapy, and forensic DNA testing all had similar origins in seemingly unrelated investigations.
