20.5 Morphological Evolution
One of the most obvious and interesting categories of evolving traits is that of organism morphology. Among animals, for example, there is great diversity in the number, kind, size, shape, and color of body parts. Since adult form is the product of embryonic development, changes in form must be the result of changes in what happens during development. Recent advances in understanding the genetic control of development (see Chapter 13) have enabled researchers to investigate the genetic and molecular bases of the evolution of animal form. We will see that some dramatic changes in animal form have a relatively simple genetic and molecular basis, while the evolution of traits governed by many “toolkit” genes involves molecular mechanisms that are distinct from those we have examined thus far. We will examine cases in which coding substitutions, gene inactivation, and regulatory sequence evolution, respectively, underlie morphological divergence.
Adaptive changes in a pigment-regulating protein
Some of the most striking and best-understood examples of morphological divergence are found in animal body-color patterns. Mammalian-coat, bird-plumage, fish-scale, and insect-wing color schemes are wonderfully diverse. Investigators have made much progress in understanding the genetic control of color formation and its role in the evolution of color differences within and between species.
In the Pinacate region of southwestern Arizona, dark rocky outcrops are surrounded by lighter-colored sandy granite (Figure 20-10). The rock pocket mouse, Chaetodipus intermedius, inhabits the Pinacate as well as other rocky areas of the Southwest. The mice found on the lava outcrops are typically dark in color, whereas those found in surrounding areas of sandy-colored granite or on the desert floor are usually light colored (Figure 20-11). Field studies suggest that color matching of coat color and environment protects mice against being seen by predators.
Figure 20-10: Contrasting colors of the pinacate desert

Figure 20-10: Lava flows in the Pinacate desert have produced outcrops of black-colored rock adjacent to sandy-colored substrates.
[Michael Nachman, University of Arizona.]
Figure 20-11: Melanism in the rock pocket mouse

Figure 20-11: Light- and dark-colored Chaetodipus intermedius from the Pinacate region of Arizona are shown on sandy-colored and dark lava-rock backgrounds.
[Michael Nachman, from M. W. Nachman et al., “The Genetic Basis of Adaptive Melanism in Pocket Mice,” Proc. Natl. Acad. Sci. USA 100, 2003, 5268-5273.]
Page 780
Figure 20-12: Amino acid replacements cluster in one part of the MC1R protein
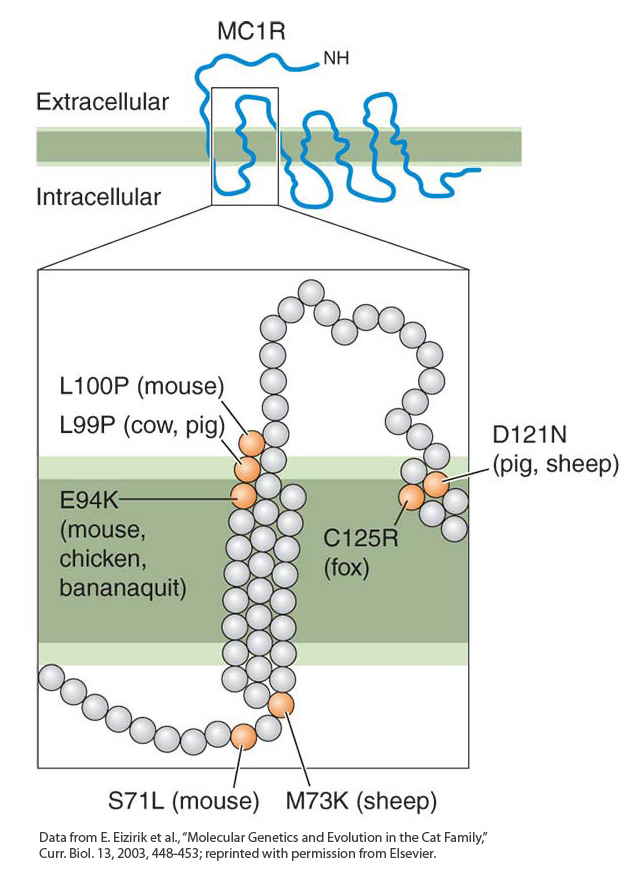
Figure 20-12: Amino acid replacements (orange circles) associated with melanism vary slightly in location in different species but are located in the same part of the MC1R protein. The upper part of the figure shows the general topology of the MC1R protein. The region in which replacements are located is enlarged in the lower part of the figure.
[Data from E. Eizirik et al., “Molecular Genetics and Evolution in the Cat Family,” Curr. Biol. 13, 2003, 448-453; reprinted with permission from Elsevier.]
The rock pocket mice give an example of melanism—the occurrence of a dark form within a population or species. Melanism is one of the most common types of phenotypic variation in animals. The dark color of the fur is due to heavy deposition of the pigment melanin, the most widespread pigment in the animal kingdom. In mammals, two types of melanin are produced in melanocytes (the pigment cells of the epidermis and hair follicles): eumelanin, which forms black or brown pigments, and phaeomelanin, which forms yellow or red pigments. The relative amounts of eumelanin and phaeomelanin are controlled by the products of several genes. Two key proteins are the melanocortin 1 receptor (MC1R) and the agouti protein. During the hair-growth cycle, the α-melanocyte-stimulating hormone (α-MSH) binds to the MC1R protein, which triggers the induction of pigment-producing enzymes. The agouti protein blocks MC1R activation and inhibits the production of eumelanin.
Michael Nachman and his colleagues examined the DNA sequences of the mc1r genes of light- and dark-colored pocket mice. They found the presence of four mutations in the mc1r gene in dark mice that cause the MC1R protein to differ at four amino acid residues from the corresponding protein in light mice. Findings from biochemical studies suggest that such mutations cause the MC1R protein to be constitutively active (active at all times), bypassing the regulation of receptor activity by the agouti protein. Indeed, mutations in mc1r are associated with melanism in all sorts of wild and domesticated vertebrates. Many of these mutations alter residues in the same part of the MC1R protein, and the same mutations have occurred independently in some species (Figure 20-12).
In many ways, we can think of these dark mice as analogs of Darwin’s finches and the lava outcrops as new “island” habitats produced by the same volcanic activity that produced the Galápagos Islands. The sandy-colored form of the mouse appears to be the ancestral type, akin to the continental ancestral finch that colonized the Galápagos. The advantage of being less visible to predators resulted in natural selection for coat color, and the invasion of the lava-rock islands by the mice led to the spread of an allele that was favored on the blackrock background and selected against on the sandy-colored background. New mutations in the mc1r gene were essential to this adaptation to the changing landscape.
Page 781
The evolution of melanism in the pocket mice illustrates how fitness depends on the conditions in which an organism lives. The new black mutation was favored on the lava outcrops but disfavored in the ancestral population living on sandy-colored terrain.
KEY CONCEPT
The relative fitness of a new variant depends on the immediate selective conditions. A mutation that may be beneficial in one population may be deleterious in another.
Gene inactivation
It has long been noted that cave-dwelling animals are often blind and uncolored. Darwin noted in The Origin of Species that “several animals belonging to the most different classes, which inhabit the caves of Carniola [in Slovenia] and Kentucky, are blind. As it is difficult to imagine that eyes, though useless, could be in any way injurious to animals living in darkness, their loss may be attributed to disuse.” 7
Figure 20-13: Evolution of albinism in blind cave fish

Figure 20-13: Surface forms of the fish Astyanu mexicanus appear normal, but cave populations, such as those from the Molino and Pachón caves in Mexico, have repeatedly evolved blindness and albinism.
[Courtesy of Richard Borowsky.]
Many species of fish that live in caves have lost their eyes and body color. Because these species belong to many different families that include surface-dwelling, eye-bearing species, the loss of eyes and pigmentation has clearly occurred repeatedly. For example, the Mexican blind cave fish (Astyonax mexicanas) belongs to the same order as the piranha and the colorful neon tetra. About 30 cave populations of fish in Mexico have lost the body color of their surface-dwelling relatives (Figure 20-13).
Genetic studies have indicated that albinism in the Pachón cave fish population is due to a single recessive mutation. Furthermore, a cross between a Molino cave individual and a Pachón cave individual produced only albino offspring, suggesting that the albinism in the two populations is due to the same genetic locus. To identify the gene responsible for albinism in the fish, researchers investigated the genotypes of fish at several pigmentation loci known to cause albinism in mice or humans. They found that one of these genes, Oca2, mapped to the albino locus. They also found that there was a perfect association between the genotype of the Oca2 locus and the phenotype of albinism in F2 offspring that were a backcross between Molino and Molino/surface F1 progeny or Pachón and Pachón/surface F1 progeny.
Further inspection of the Oca2 gene revealed that the Pachón population was homozygous for a deletion that extended from an intron through most of an exon and that the Molino population was homozygous for the deletion of a different exon. Functional analyses proved that each deletion in the Oca2 gene caused loss of Oca2 function.
The identification of different lesions in the Oca2 gene of the two cave populations indicates that albinism evolved separately in the two cave populations. There is also evidence that a third cave population carries yet a third, distinct Oca2 mutation. It is known from other vertebrates that albinism can evolve through mutations in other genes. What might account for the repeated inactivation of the Oca2 gene? There are two likely explanations. First, Oca2 mutations appear to cause no serious collateral defects other than loss of pigmentation and vision. Some other pigmentation genes, when mutated in fish, cause dramatic reductions in viability. The effects of Oca2 mutations appear, then, to be less pleiotropic and have effects on overall fitness that are less harmful than those of mutations in other fish pigmentation genes. Second, the Oca2 locus is very large, spanning some 345 kb in humans and containing 24 exons. It presents a very large target for random mutations that would disrupt gene function; Oca2 mutations are therefore more likely to arise than are mutations at smaller loci.
Page 782
The loss of gene function is not what we usually think about when we think about evolution. But gene inactivation is certainly what we should predict to happen when selective conditions change or when populations or species shift their habitats or lifestyles and certain gene functions are no longer necessary.
7 C. Darwin, On the Origin of Species by Means of Natural Selection, p. 137 John Murray, London, 1859.
KEY CONCEPT
Gene-inactivating mutations may occur and rise to high frequency when habitat or lifestyle changes relax natural selection on traits and underlying gene functions.
Regulatory-sequence evolution
Figure 20-14: Wing spots on fruit flies

Figure 20-14: Drosophila melanogaster males lack wing spots (top), whereas Drosophila biarmipes males (bottom) have dark wing spots that are displayed in a courtship ritual. This simple morphological difference is due to differences in the regulation of pigmentation genes.
[Nicolas Gompel.]
As discussed above, a major constraint on gene evolution is the potential for harmful side effects caused by mutations in coding regions that alter protein function. These effects can be circumvented by mutations in regulatory sequences, which play a major role in the evolution of gene regulation and body form.
The examples of body-coloration evolution we have looked at thus far have the coat or scale pattern changing over the entire body. The evolution of solid black or entirely unpigmented body coloration can arise through mutations in pigmentation genes. However, many color schemes are often made up of two or more colors in some spatial pattern. In such cases, the expression of pigmentation genes must differ in areas of the body that will be of different colors. In different populations or species, the regulation of pigmentation genes must evolve by some mechanism that does not disrupt the function of pigmentation proteins.
The species of the fruit-fly genus Drosophila display extensive diversity of body and wing markings. A common pattern is the presence of a black spot near the tip of the wing in males (Figure 20-14). The production of the black spots requires enzymes that synthesize melanin, the same pigment made in pocket mice. Many genes controlling the melanin synthesis pathway have been well studied in the model organism Drosophila melanogaster. One gene is named yellow because mutations in the gene cause darkly pigmented areas of the body to appear yellowish or tan. The yellow gene plays a central role in the development of divergent melanin patterns. In species with spots, the Yellow protein is expressed at high levels in wing cells that will produce the black spot, whereas in species without spots, Yellow is expressed at a low level throughout the wing blade (Figure 20-15a).
Figure 20-15: Changes in regulatory sequences can underlie evolutionary differences
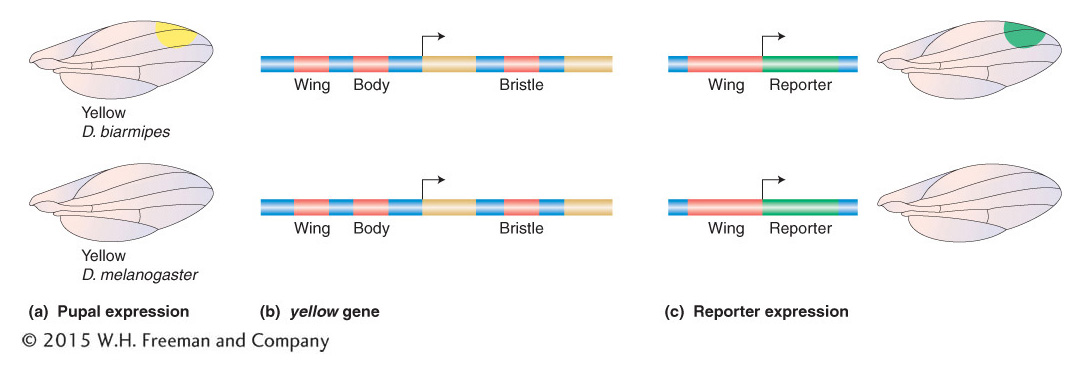
Figure 20-15: The evolution of gene regulation and morphology in the case shown is due to evolution in cis-acting regulatory sequences. (a) In spotted fruit flies, the Yellow pigmentation protein is expressed at high levels in cells that will produce large amounts of melanin. (b) The yellow locus of Drosophila species contains several discrete cis-acting regulatory elements (red) that govern yellow transcription in different body parts. Exons are shown in gold. Arrows indicate the point of the start and direction of transcription of the gene. (c) The “wing” regulatory element from D. biarmipes drives reporter-gene expression in a spot pattern in the developing wing, whereas the homologous element from the unspotted D. melanogaster does not drive a spot pattern of reporter expression. This difference in wing cis-acting-regulatory-element activities demonstrates that changes in the cis-acting-regulatory-element function underlie differences in Yellow expression and pigmentation between the two species.
The difference in Yellow expression between spotted and unspotted species could be due to differences in how the yellow gene is regulated in the two species. Either or both of two possible mechanisms could be at play: the species could differ in the spatial deployment of transcription factors that regulate yellow (that is, changes in trans-acting sequences to the yellow gene), or they could differ in cis-acting regulatory sequences that govern how the yellow gene is regulated. To examine which mechanisms are involved, investigators examined the activity of yellow cis-acting regulatory sequences from different species by placing them upstream of a reporter gene and introducing them into D. melanogaster.
The yellow gene is regulated by an array of separate cis-acting regulatory sequences that govern gene transcription in different tissues and cell types and at different times in development (Figure 20-15b). These regulatory sequences include those controlling transcription in the larval mouthparts, the pupal thorax and abdomen, and the developing wing blade. It was discovered that, whereas the wing-blade cis-acting regulatory element from unspotted species drives low-level expression of a reporter gene across the wing blade, the corresponding element from a spotted species, such as D. biarmipes or D. elegans, drives a high level of reporter expression in a spot near the tip of the wing (Figure 20-15c). These observations show that changes in sequence and function of a cis-acting regulatory element are responsible for the change in yellow regulation and contribute to the origin of the wing spot. It was demonstrated that the cis-acting regulatory element of the spotted species has acquired binding sites for transcription factors that now drive high levels of gene transcription in a spot pattern in the developing wing.
Page 783
Thus, evolutionary changes in cis-acting regulatory sequences play a critical role in the evolution of body form. The location of change in the regulatory sequence rather than the gene itself can be best explained in light of the many different effects that can appear as the result of a coding mutation in a “toolkit” gene. In this instance, the yellow gene is highly pleiotropic: it is required for the pigmentation of many structures and for functions in the nervous system as well. A coding mutation that alters Yellow protein activity would alter Yellow activity in all tissues, which might have a negative consequence for fitness. However, because individual cis-acting regulatory sequences usually affect only one aspect of gene expression, mutations in these sequences provide a mechanism for changing one aspect of gene expression while preserving the role of protein products in other developmental processes.
KEY CONCEPT
Evolutionary changes in cis-acting regulatory sequences play a critical role in the evolution of gene expression. They circumvent the pleiotropic effects of mutations in the coding sequences of genes that have multiple roles in development.
Loss of characters through regulatory-sequence evolution
Morphological characters may be lost as well as gained as the result of adaptive changes in cis-acting regulatory sequences. If there is no selective pressure to maintain a character, it can be lost over time. But some losses are beneficial because they facilitate some change in lifestyle. Hind limbs, for example, have been lost many times in vertebrates—in snakes, lizards, whales, and manatees—as these organisms adapted to different habitats and means of locomotion. Evolutionary changes in cis-acting regulatory sequences are also linked to these dramatic changes.
Page 784
The evolutionary forerunners of the hind limbs of four-legged vertebrates are the pelvic fins of fish. Dramatic differences in pelvic-fin anatomy have evolved in closely related fish populations. The three-spine stickleback fish occurs in two forms in many lakes in North America—an open-water form that has a full spiny pelvis and a shallow-water, bottom-dwelling form with a dramatically reduced pelvis and spines. In open water, the long spines help protect the fish from being swallowed by larger predators. But on the lake bottom, those spines are a liability because they can be grasped by dragonfly larvae that feed on the young fish (Figure 20-16a and 20-16b).
Figure 20-16: Spine loss is due to the mutation of a regulatory sequence
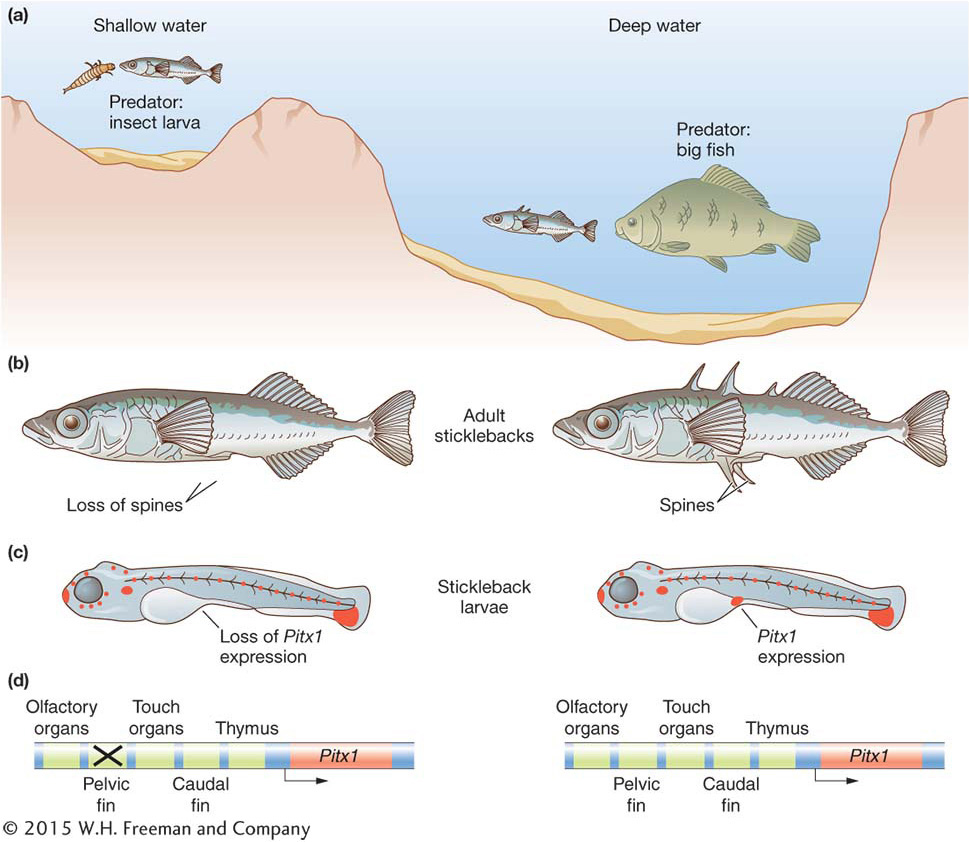
Figure 20-16: Deletions within a Pitx1 cis-regulatory element underlie the adaptive evolution of the pelvic skeleton of stickleback fish. (a) One form of the three-spine stickleback fish inhabits shallow water, and a different form inhabits open water. (b) The shallow-water form has a reduced pelvic skeleton (left) relative to the open-water form (right). (c) This reduction is due to the selective loss of expression of the Pitx1 gene (orange) from the pelvic fin bud during development of the stickleback larvae (compare left and right stickleback larvae). (d) The loss of Pitx1 expression in turn is due to the mutation of an enhancer of the Pitx1 gene specific to the pelvic fin (X marks the mutated enhancer). Other enhancers of the Pitx1 gene, which control expression of the gene elsewhere in the developing body, are unaffected and function similarly in both forms of the fish.
The differences in pelvic morphology have evolved repeatedly in just the past 10,000 years, since the recession of the glaciers of the last ice age. Many separate lakes were colonized by long-spined oceanic sticklebacks, and forms with reduced pelvic spines evolved independently several times. Because the fish are so closely related and interbreed in the laboratory, geneticists can map the genes involved in the reduction of the pelvis. David Kingsley’s group at Stanford University along with Dolph Schluter’s group at the University of British Columbia mapped one major factor involved in pelvic differences to the Pitx1 gene, which encodes a transcription factor. Like most other developmental toolkit genes, the Pitx1 gene has several distinct functions in fish development. However, in the form of the stickleback with a reduced pelvis, its expression is lost from the area of the developing fish embryo that will give rise to the pelvic-fin bud and spines (see Figure 20-16).
The fact that the difference in pelvic morphology between the two forms mapped to the Pitx1 locus and was associated with the loss of gene expression suggested that changes in Pitx1 regulatory sequences were responsible for the difference in phenotypes. Like most pleiotropic toolkit genes, the expression of the Pitx1 gene in different parts of the developing fish is controlled by separate cis-acting regulatory elements. Frank Chan and colleagues demonstrated that the regulatory element that controls Pitx1 expression in the developing pelvis has been inactivated by large deletion mutations in multiple, independent populations of pelvic-reduced fish (Figure 20-16c and 20-16d). Furthermore, it was observed that heterozygosity was reduced around the cis-acting sequences controlling pelvic expression relative to other nearby sequences. This observation is consistent with the deletion allele being favored by natural selection acting on the bottom-dwelling, pelvic-reduced form.
Thus, these findings further illustrate how mutations in regulatory sequences circumvent the pleiotropic effects of coding mutations in toolkit genes and that adaptive changes in morphology can be due to the loss as well as the gain of gene expression during development.
KEY CONCEPT
Adaptive changes in morphology can result from inactivation of regulatory sequences and loss of gene expression as well as the modification of regulatory sequences and the gain of gene expression.Circumventing the potentially harmful side effects of coding mutations is a very important factor in explaining why evolution acts by generating new roles for transcription factors that may regulate dozens to hundreds of target genes. Changes in the coding sequences of a transcription factor—for example, the DNA-binding domain—may affect all target genes, with catastrophic consequences for the animal. The constraint on the coding sequences of highly pleiotropic proteins, with many functions, explains the extraordinary conservation of the DNA-binding domains of Hox proteins (see Figure 13-8) and many other transcription factors over vast expanses of evolutionary time. But, although the proteins’ biochemical functions are constrained, their regulation does diverge. The evolution of the expression patterns of Hox and other toolkit genes plays a major role in the evolution of body form.
Page 785
Regulatory evolution in humans
Regulatory evolution is not limited to genes affecting development. The level, timing, or spatial pattern of the expression of any gene may vary within populations or diverge between species. For example, as noted earlier (see Chapter 18), the frequencies of alleles at the Duffy blood-group locus vary widely in human populations. The Duffy locus (denoted Fy) encodes a glycoprotein that serves as a receptor for multiple intercellular signaling proteins. In sub-Saharan Africa, most members of indigenous populations carry the Fynull allele. Individuals with this allele do not express any of the Duffy glycoprotein on red blood cells, although the protein is still being produced in other cell types. How and why is the Duffy glycoprotein lacking on these individuals’ red blood cells?
The molecular explanation for the lack of Duffy glycoprotein expression on red blood cells is the presence of a point mutation in the promoter region of the Duffy gene at position –46. This mutation lies in a binding site for a transcription factor specific to red blood cells called GATA1 (Figure 20-17). Mutation of this site abolishes the activity of a Duffy gene enhancer in reporter-gene assays.
Figure 20-17: Mutation in a regulatory element increases resistance to malaria
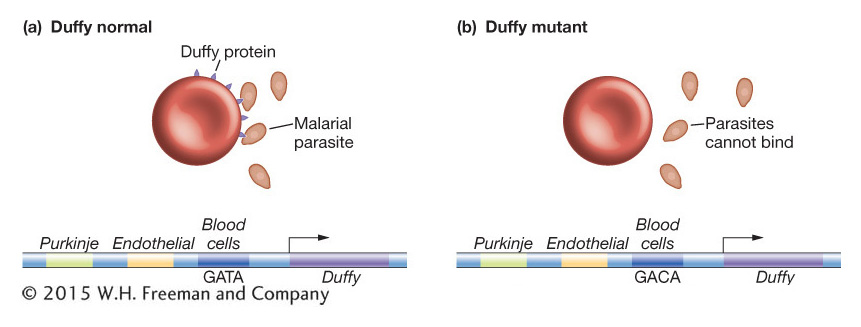
Figure 20-17: A regulatory mutation in a human Duffy gene enhancer is associated with resistance to malaria. (a) The Duffy protein (dark blue) is typically expressed on blood cells as well as on Purkinje cells in the brain and endothelial cells. (b) A high proportion of West Africans lack Duffy expression on their red blood cells due to a mutation in a blood-cell enhancer (the GATA sequence is mutated to GACA). Since the Duffy protein is part of the receptor for the P. vivax malarial parasite (orange), individuals with the regulatory mutation are resistant to infection but have normal Duffy expression elsewhere in the body.
An evolutionary explanation suggests that the lack of Duffy glycoprotein expression on red blood cells among Africans is the result of natural selection favoring resistance to malarial infection. The malarial parasite Plasmodium vivax is the second most prevalent form of malarial parasite in most tropical and subtropical regions of the world but at present is absent from sub-Saharan Africa. The parasite gains entry to red blood cells and red-blood-cell precursors by binding to the Duffy glycoprotein (see Figure 20-17). The very high frequency of Fynull homozygotes in Africa prevents P. vivax from being common there. Moreover, if we suppose that P. vivax was common in Africa in the past, then the Fynull allele would have been selected for.
Page 786
The complete absence of Duffy protein on the red blood cells of a large subpopulation raises the question of whether the Duffy protein has any necessary function, because it is apparently dispensable. But it is not the case that these individuals lack Duffy protein expression altogether. The protein is expressed on endothelial cells of the vascular system and the Purkinje cells of the cerebellum. As with the evolution of Yellow expression in wing-spotted fruit flies and of Pitx expression in stickleback fish, the regulatory mutation at the Fy locus allows one aspect of gene expression (in red blood cells) to change without disrupting others (see Figure 20-17).
Modifications to coding and regulatory sequences are common means to evolutionary change. They illustrate how diversity can arise without the number of genes in a species changing. However, larger-scale mutational changes can and do happen in DNA that result in the expansion of gene number, and this expansion provides raw material for evolutionary innovation.







