12-2 The Chemical Senses
Chemical reactions are central to nervous system activity, and chemosignals (chemical signals) play a central role in motivated and emotional behavior. Mammals identify group members by odor, mark their territory with urine and other odorants, identify favorite and forbidden foods by taste, and form associations among odors, tastes, and emotional events.
Olfaction
Olfaction is the most puzzling sensory system. We can discriminate thousands of odors, yet we have great difficulty finding words to describe what we smell. We may like or dislike smells or compare one smell to another, but we lack a vocabulary for olfactory perceptions.
Wine experts rely on olfaction to tell them about wines, but they must learn to use smell to do so. Training courses in wine sniffing typically run one full day per week for a year, and most participants still have great difficulty passing the final test. This degree of difficulty contrasts with that of vision and audition, senses designed to analyze the specific qualities of sensory input, such as pitch in audition or color in vision. In contrast, olfaction seems designed to discriminate whether information is safe or familiar—
Receptors for Smell
Conceptually, identifying chemosignals is similar to identifying other sensory stimuli (light, sound, touch). But rather than converting physical energy such as light or sound waves into receptor potentials, scent interacts with chemical receptors. This constant chemical interaction must be tough on the receptors: in contrast with receptors for light, sound, and touch, chemical receptors are constantly being replaced. The life of an olfactory receptor neuron is about 60 days.
The receptor surface for olfaction, illustrated in Figure 12-3, is the olfactory epithelium in the nasal cavity. The epithelium is composed of receptor cells and support cells. Each receptor cell sends a process ending in 10 to 20 cilia into a mucous layer, the olfactory mucosa. Chemicals in the air we breathe dissolve in the mucosa to interact with the cilia. If an olfactory chemosignal affects the receptors, metabotropic activation of a specific G protein leads to an opening of sodium channels and a change in membrane potential.
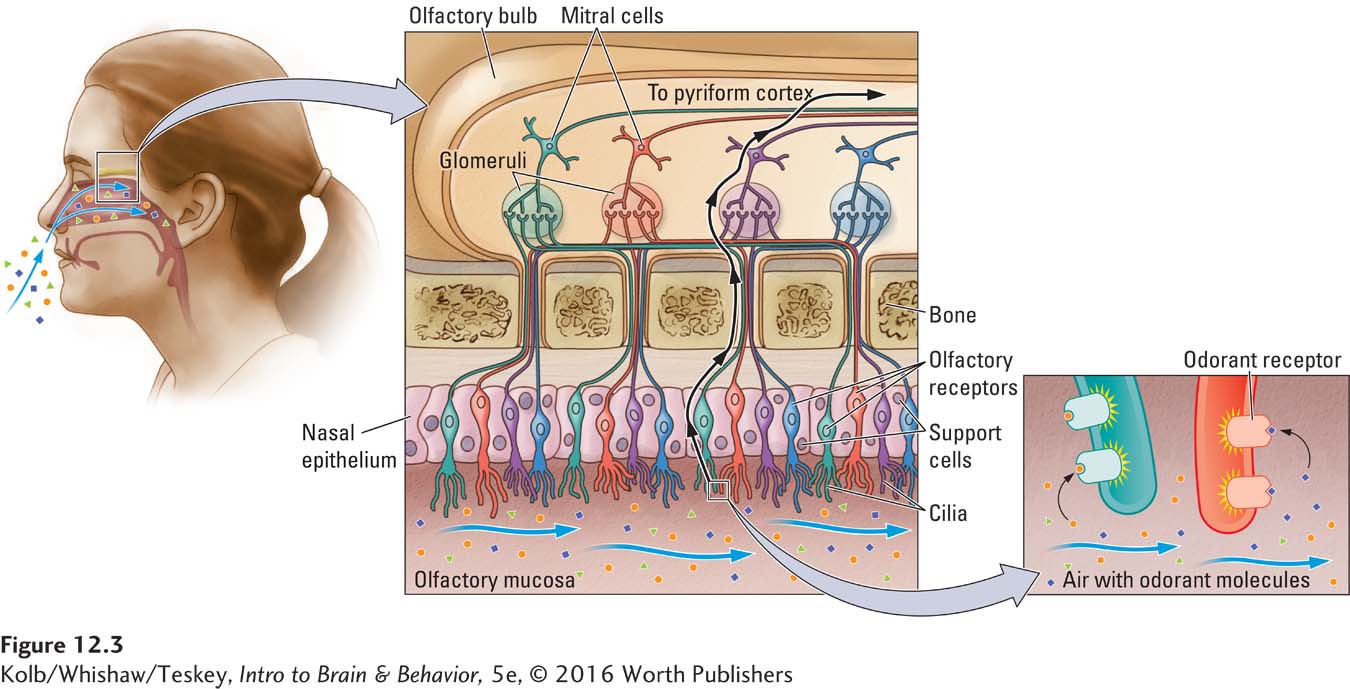
Figure 5-15A illustrates activity in such a metabotropic receptor.
The epithelial receptor surface varies widely across species. In humans, this area is estimated to range from 2 to 4 square centimeters; in dogs, about 18 square centimeters; and in cats, about 21 square centimeters. No wonder our sensitivity to odors is less acute than that of dogs and cats: they have 10 times as much receptor area as humans have! Roughly analogous to the tuning characteristics of cells in the auditory system, olfactory receptor neurons in vertebrates do not respond to specific odors but rather to a range of odors.
How does a limited number of receptor types allow us to smell many different odors? The simplest explanation is that any given odorant stimulates a unique pattern of receptors, and the summed activity or pattern of activity produces our perception of a particular odor. Analogously, the visual system enables us to identify several million colors with only three receptor types in the retina: the summed activity of the three cones leads to our richly colored life.
A fundamental difference, however, is that the olfactory system is estimated to contain about 400 kinds of receptors compared with 4 (rods plus the cones) in the visual system. Some researchers propose that humans can discriminate up to 1 trillion smells. At present the true number is unknown but likely more than commonly believed (Gerkin & Castro, 2015).
Olfactory Pathways
Olfactory receptor cells project to the olfactory bulb, ending in ball-
Accessory Olfactory System
A unique class of odorants is pheromones, biochemicals released by one animal that act as chemosignals and can affect the physiology or behavior of another animal of the same species. For example, Karen Stern and Martha McClintock (1998) found that when women reside together, their estrous cycles begin to synchronize. Furthermore, the researchers found that the synchronization of menstrual cycles, a phenomenon called the Whitten effect, is stimulated by the odor of male urine.
Pheromones appear able to affect more than sex-
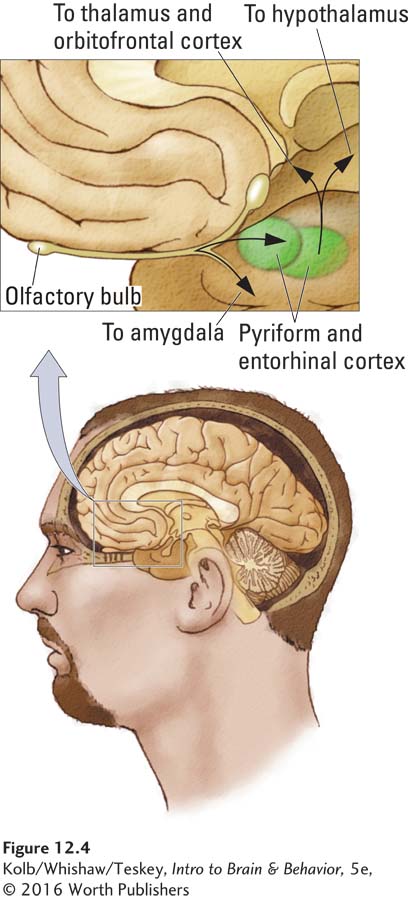
Pheromones are unique odors because they are detected by a special olfactory receptor system, the vomeronasal organ, which is made up of a small group of sensory receptors connected by a duct to the nasal passage. The receptor cells in the vomeronasal organ send their axons to the accessory olfactory bulb, which lies adjacent to the main olfactory bulb. The vomeronasal organ connects primarily with the amygdala and hypothalamus, via which it probably plays a role in reproductive and social behavior.
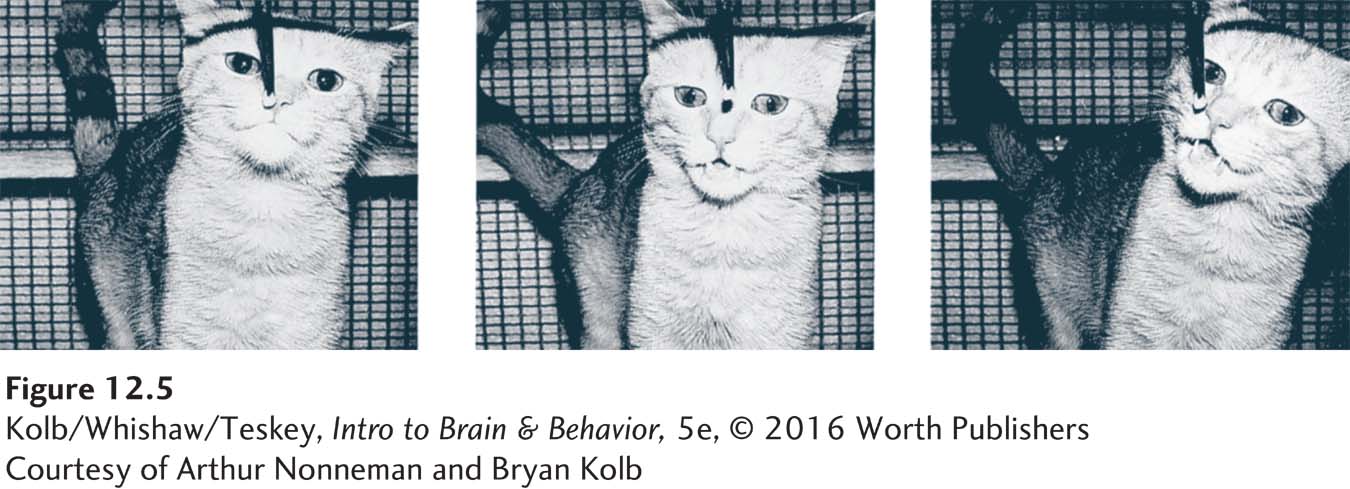
It is likely that the vomeronasal organ participates not in general olfactory behavior but rather in the analysis of pheromones, such as those in urine. You may have seen bulls or cats engage in a behavior known as flehmen, illustrated in Figure 12-5. When exposed to novel urine from a cat or human, cats raise their upper lip to close off the nasal passages and suck air into the mouth. The air flows through the duct on the roof of the mouth en route to the vomeronasal organ.
Human Olfactory Processing
Our ability to distinguish a surprisingly large number of odors upends the common misperception that people have a miserable sense of smell relative to other mammals. Still, our threshold for detecting many smells is certainly inferior to that of our pet dogs, cats, and horses. Yet humans have a surprisingly acute sensitivity to behaviorally significant smells.
Several studies show convincingly that people can identify their own odor, the odor of kin versus not-
First, the brain analyzes common odors and body odors differently. Although both activate primary olfactory regions, body odors also activate structures not previously believed to participate in olfactory processing, including the posterior cingulate cortex, occipital cortex, and anterior cingulate cortex—
The insula contains regions related to language, taste perception, and social cognition.
Second, smelling a stranger’s odor activates the amygdala and insular cortex, similar to activation observed for fearsome visual stimuli such as masked or fearsome faces. The investigators also asked participants to rate the intensity and pleasantness of odors; they found that strangers’ odors were rated as stronger and less pleasant than those of familiar persons.
Lundstrom and colleagues conclude that processing body odors is mostly unconscious. It represents an automatic process that matches odors to a learned library of smells. Similar unconscious processes seem to occur during visual and auditory information processing and to play an important role in our emotional reactions.
Gustation
Research reveals significant differences in taste preferences both between and within species. Humans and rats like sucrose and saccharin solutions, but dogs reject saccharin and cats are indifferent to both, inasmuch as they do not detect sweetness at all. The failure of cats to taste sweet may not be surprising: they are pure carnivores, and nothing that they normally eat is sweet.
Within the human species, clear differences in taste thresholds and preferences are obvious. An example is the preference for or dislike of bitter tastes—
Sensitivity to bitterness is related to genetic differences in the ability to detect a specific bitter chemical (6-
Valerie Duffy and her colleagues (2010) investigated sensitivity to quinine (usually perceived as bitter) in participants who were assessed for the TAS2R38 genotype. They estimated participants’ taste bud density by counting the number of papillae (the little bumps on the tongue). Quinine was reported as more bitter to those who tasted PROP as very bitter or to those who had more taste buds. Thus, detection of bitterness is related both to TAS2R38 and to tongue anatomy.
Nontasters, either by genotype or phenotype (for few taste buds), reported greater consumption of vegetables, both bitter and not. Nontasters with higher numbers of taste buds reported eating about 25% more vegetables than the other groups. These data suggest that genetic variation in taste can explain differences in overall consumption of all types of vegetables. It also suggests that persuading supertasters to eat more healthy foods—
Differences in taste thresholds also emerge as we age. Children are much more responsive to taste than adults and are often intolerant of spicy foods because they have more taste receptors than adults have. It is estimated that by age 20, humans have lost at least 50 percent of their taste receptors. No wonder children and adults have different food preferences.
Receptors for Taste
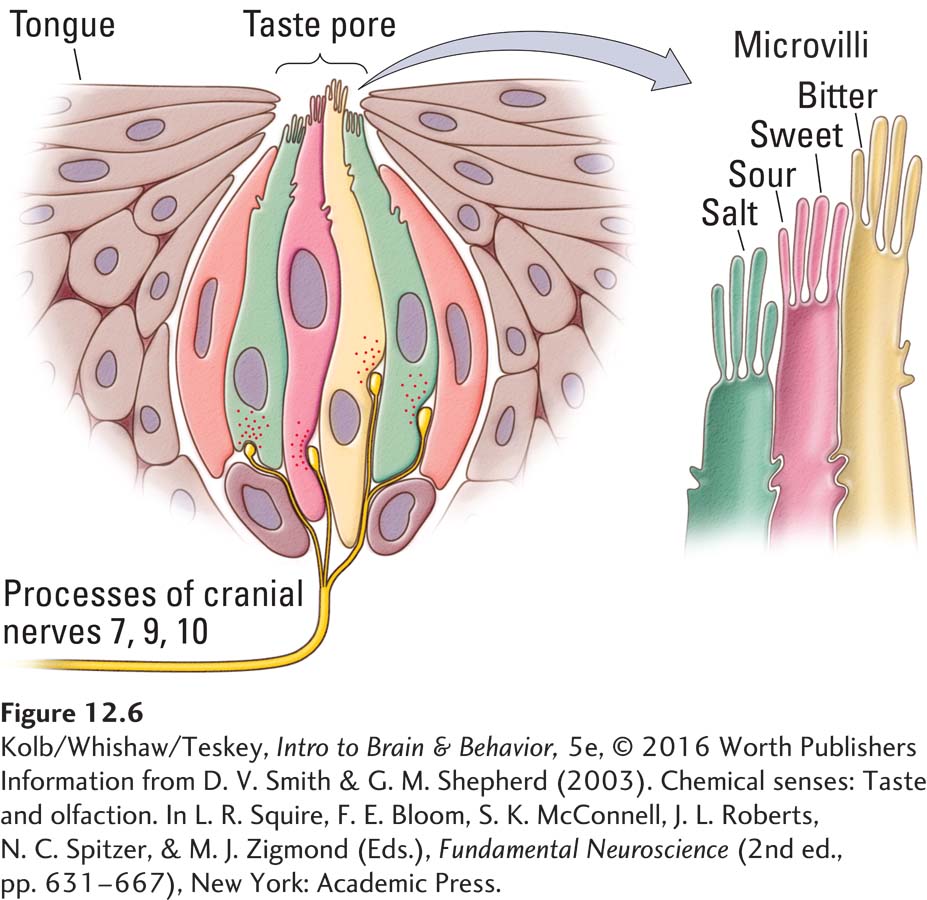
Taste receptors are found in taste buds on the tongue, under the tongue, on the soft palate on the roof of the mouth, on the sides of the mouth, and at the back of the mouth on the nasopharynx. Each of the five taste receptor types responds to a different chemical component in food. The four most familiar are sweet, sour, salty, and bitter. The fifth type, called the umami receptor, is especially sensitive to glutamate, a neurotransmitter molecule, and perhaps to nucleotides. (Nucleotide bases are the structural units of nucleic acids such as DNA and RNA.)
Taste receptors are grouped into taste buds, each containing several receptor types, as illustrated in Figure 12-6. Gustatory stimuli interact with the receptor tips, the microvilli, to open ion channels, leading to changes in membrane potential. At its base, the taste bud contacts the branches of afferent cranial nerve 7 (facial), 9 (glossopharyngeal), or 10 (vagus).
Gustatory Pathways
Cranial nerves 7, 9, and 10 form the main gustatory nerve, the solitary tract. On entering the brainstem, the tract splits, as illustrated in Figure 12-7. One route (traced in red) travels through the posterior medulla to the ventroposterior medial nucleus of the thalamus. This nucleus in turn sends out two pathways, one to the primary somatosensory cortex (S1) and the other to the primary gustatory cortex of the insula, a region just rostral to the secondary somatosensory cortex (S2).
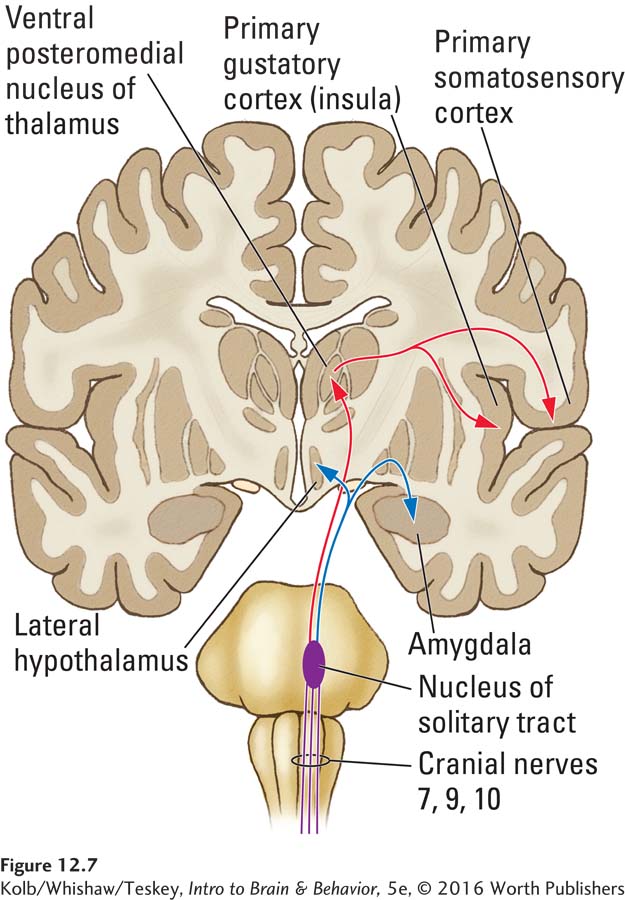
The gustatory region in the insula is dedicated to taste, whereas S1 is also responsive to tactile information and is probably responsible both for localizing tastes on the tongue and for our reactions to a food’s texture. The gustatory cortex sends a projection to the orbital cortex in a region near the input from the olfactory cortex. Neuroimaging studies suggest that the mixture of olfactory and gustatory input in the orbital cortex gives rise to our perception of flavor. Ambience, including music and light, also affects this region of orbital cortex, increasing blood flow and so enhancing our experience of flavor.
A meta-
The second pathway from the gustatory nerve (shown in blue in Figure 12-7) projects via the nucleus of the solitary tract in the brainstem to the hypothalamus and amygdala. Researchers hypothesize that these inputs somehow participate in feeding behavior, possibly evaluating the pleasantness and strength of flavors.
12-2 REVIEW
The Chemical Senses
Before you continue, check your understanding.
Question 1
The receptor surface for olfaction is the _______________.
Question 2
Olfactory and gustatory pathways eventually merge in the orbitofrontal cortex, leading to the perception of ________________.
Question 3
Chemosignals that convey information about the sender are called __________________.
Question 4
The perception of bitterness is related to both the _______________ and the __________________.
Question 5
How do a relatively limited number of receptor types allow us to smell a trillion different odors?
Answers appear in the Self Test section of the book.