13-5 Neural Bases of Sleep
The idea that the brain contains a sleep-
The hormone melatonin, secreted from the pineal gland during the dark phase of the light–
Some observations suggest that it is not a circulating bloodstream compound that produces sleep. In dolphins and birds only one brain hemisphere sleeps at a time. This ability presumably allows an animal’s other hemisphere to remain behaviorally alert. More important, it suggests that sleep is produced by the action of some brain region, and in these species, within each hemisphere.
In this section we consider the neural mechanisms that regulate sleep. First we examine evidence that the activity of a slave oscillator of the suprachiasmatic nucleus produces sleep (see Figure 13-8). Second we look at evidence that a number of brainstem nuclei control the various events associated with sleep, including those associated with REM and NREM sleep.
Reticular Activating System and Sleep
A pioneering experiment by Giuseppe Moruzzi and Horace Magoun (1949) began to answer the question of which brain areas regulate sleep. The experimenters were recording the cortical EEG from anesthetized cats while electrically stimulating the cats’ brainstem. They were surprised to find that, in response to the electrical stimulation the large, slow delta EEG typical of anesthesia is replaced by the low-
The beta EEG activity outlasted the stimulation period, demonstrating that the effect was not simply due to the online activity of neurons in the region of the stimulating electrode but could be maintained by these neurons independent of the stimulation. During the “waking period” the anesthetized cat did not become behaviorally aroused, but its cortical EEG appeared to indicate that it was awake. In a sleeping cat the same stimulation did lead to waking.
Subsequent experiments show that a waking EEG and waking behavior can be induced from a large neural area running through the center of the brainstem. Anatomically this area is composed of a mixture of cell nuclei and nerve fibers that form a reticulum. Moruzzi and Magoun named this brainstem area the reticular activating system (RAS) and proposed that it is responsible for sleep–
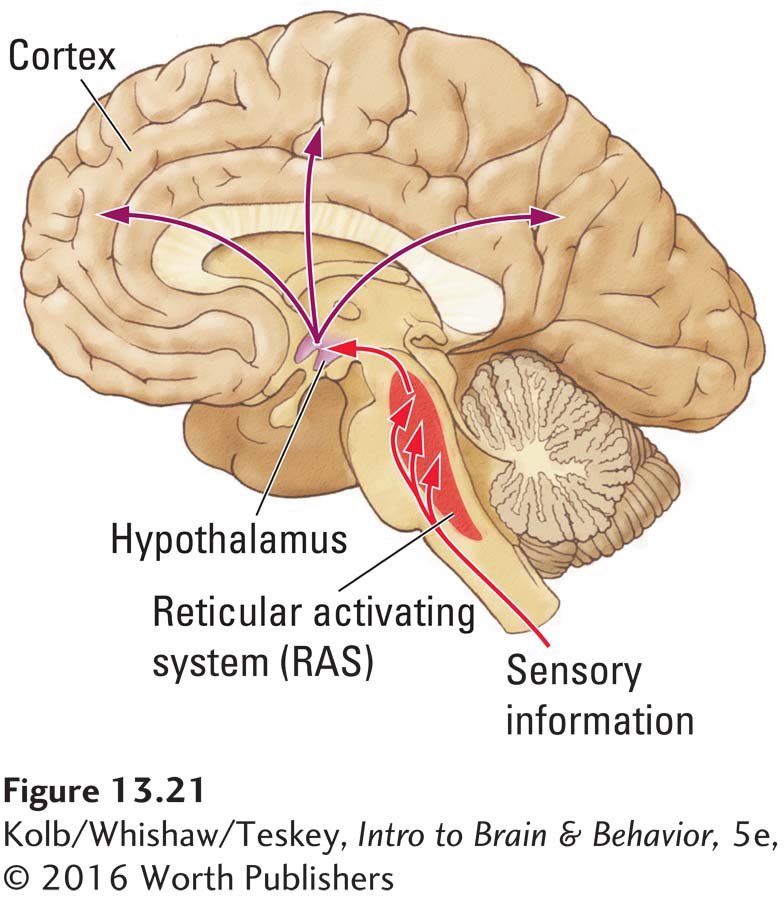
If someone disturbs you when you are asleep, you usually wake up. To explain that their experimentally induced effect did not result simply from sensory stimulation, Moruzzi and Magoun cut the brainstem just behind the RAS, severing its incoming sensory pathways. RAS stimulation still produced a desynchronized EEG. Thus, the RAS is the source of waking, and sensory stimulation produces waking because it activates RAS neurons.
The idea that the brainstem participates in waking behavior helps to explain why brainstem damage can result in coma, a prolonged state of deep unconsciousness resembling sleep. In one well-
Neural Basis of EEG Changes Associated with Waking
Figure 5-17 summarizes the major neural activating systems and their functions.
Research built on those pioneering studies of the RAS has since revealed that many neural systems in the brainstem play a role in sleeping and waking behavior. Case Vanderwolf and his coworkers (Vanderwolf, 2002) showed that at least two brainstem systems influence waking EEG. Figure 13-22 illustrates their locations in the rat brain.
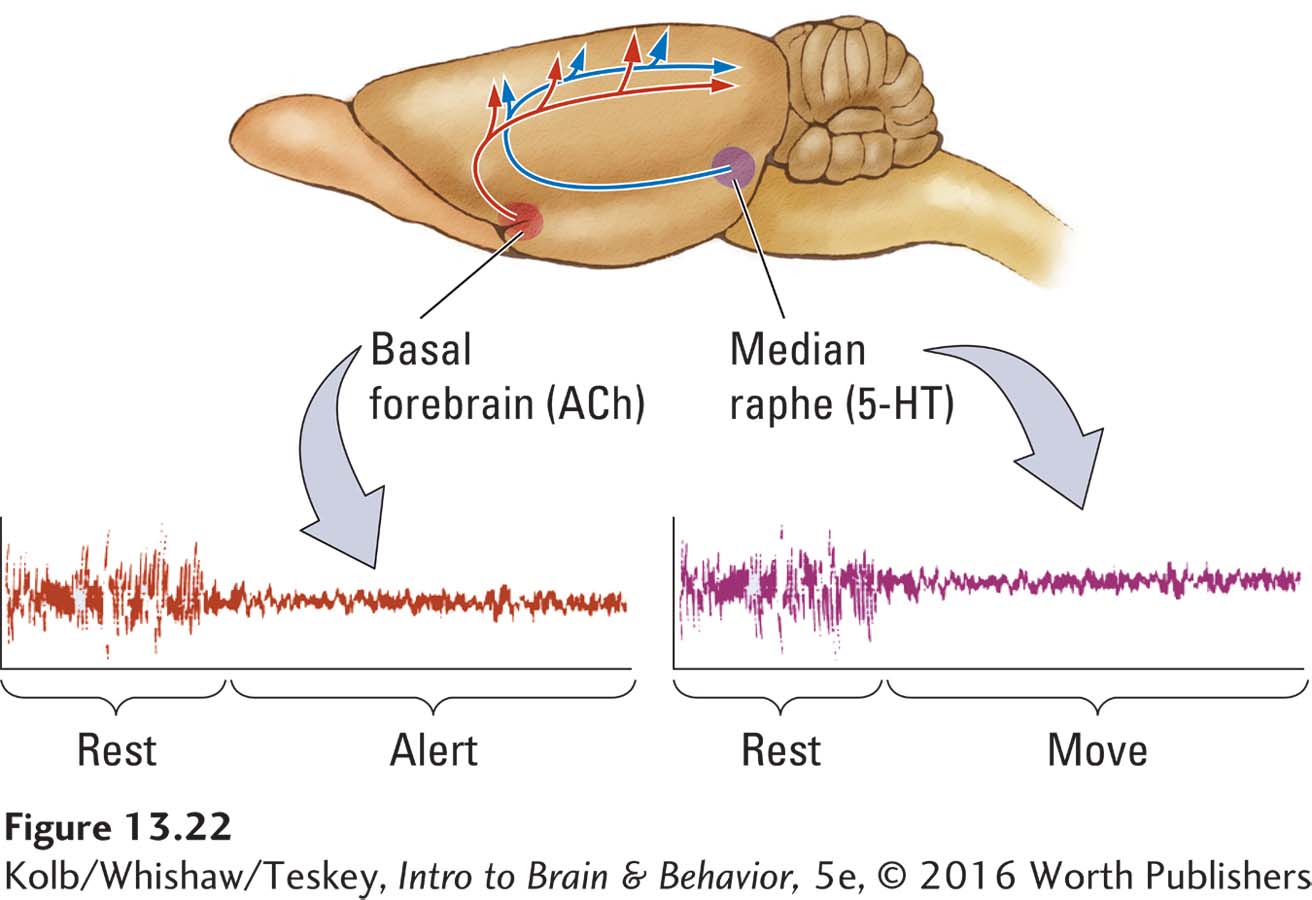
The basal forebrain contains large cholinergic cells. These neurons secrete acetylcholine (ACh) from their terminals onto cortical neurons to stimulate a waking EEG (beta rhythm). The midbrain structure the median raphe contains serotonin (5-
Although the two pathways produce similar patterns of waking EEG activity, their relations to behavior differ. If the activity of the cholinergic projection is blocked by drugs or by lesions to the cells of the basal forebrain, the waking EEG normally recorded from an immobile rat is replaced by EEG activity resembling that of NREM sleep. Only if the rat walks or is otherwise active is a waking EEG obtained from the cortex. These findings, graphed in Figure 13-22, suggest that the cholinergic EEG is responsible for waking associated with being alert yet still, whereas the serotonergic activation is responsible for the waking EEG associated with movement.
Neither the basal forebrain system nor the median raphe system is responsible for behavior. In fact, if both structures are pharmacologically or surgically destroyed, a rat can still stand and walk around. Its EEG, however, permanently resembles that of a sleeping animal.
As long as one activating system is producing a waking EEG, rats can learn simple tasks. If both systems are destroyed, an animal, although still able to walk around, is no longer able to learn or display intelligent behavior. In a sense the cortex is like a house in which the lights are powered by two separate sources: both must fail for the house to be left in darkness, but if at least one source is operating, the lights stay on.
These experimental results suggest that the RAS produces its arousal effects by influencing activity in these two pathways, which then produce EEG events associated with waking. In humans the basal forebrain and median raphe likely produce the same two desynchronized EEG patterns that they produce in rats. Consequently, when we are alert and still, cholinergic neurons are active; when we move, serotonin neurons also are active.
Perhaps when you have felt sleepy, say in a class or behind the wheel of a car, you may have been able to wake yourself up by moving—
Neural Basis of REM Sleep
Barbara Jones (1993) and her colleagues described a group of cholinergic neurons known as the peribrachial area that contributes to REM sleep. This area is located in the dorsal brainstem just anterior to the cerebellum (Figure 13-23). Jones selectively destroyed peribrachial cells by spraying them with the neurotoxin kainic acid. She found that REM sleep was drastically reduced. This result suggests that the peribrachial area contributes to REM sleep and REM-
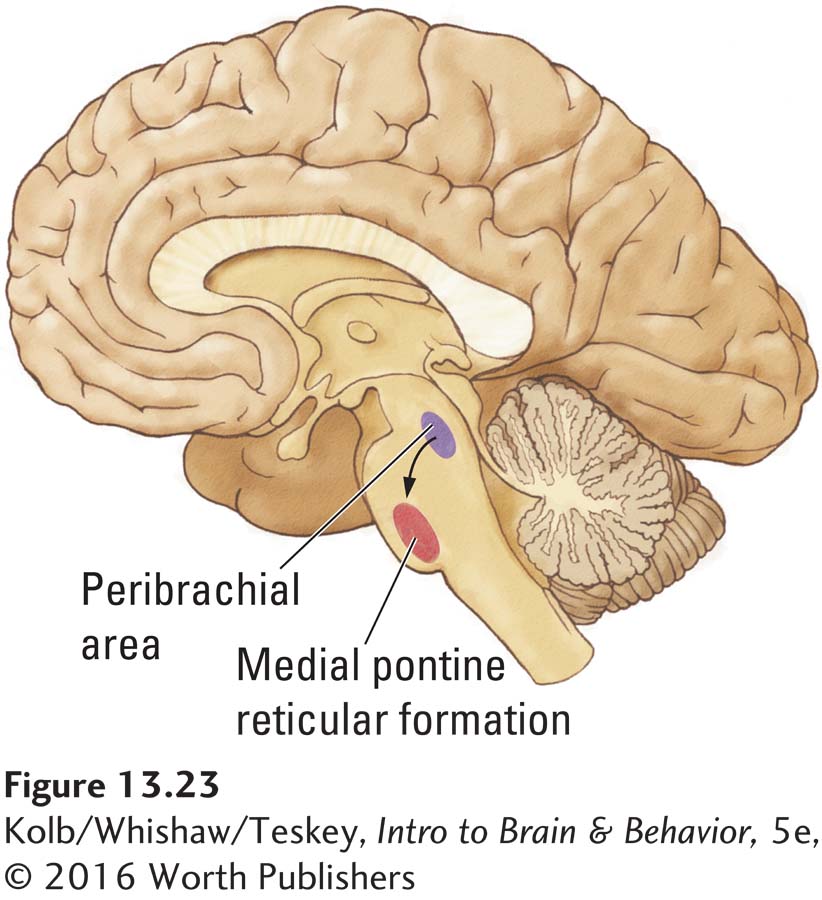
The peribrachial area extends into a more ventrally located nucleus called the medial pontine reticular formation (MPRF), shown in Figure 13-24 charts an explanation showing how other REM-
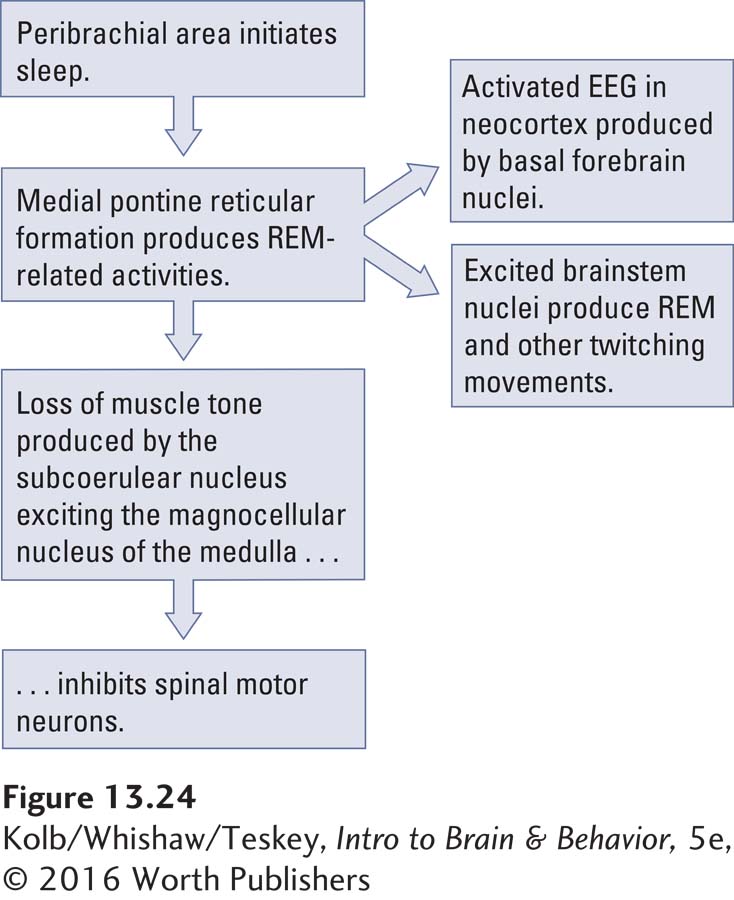
The peribrachial area initiates REM sleep by activating the medial pontine reticular formation.
The MPRF sends projections to excite basal forebrain cholinergic neurons, resulting in an activated EEG recorded from the cortex.
The MPRF also excites brainstem motor nuclei to produce rapid eye movements and other twitches.
The atonia of REM sleep is produced by the MPRF through a pathway that sends input to the subcoerulear nucleus located just behind it.
The subcoerulear nucleus excites the magnocellular nucleus of the medulla, which sends projections to the spinal motor neurons to inhibit them so that paralysis is achieved during the REM sleep period.
In support of such a neural arrangement French researcher Michel Jouvet (1972) observed that cats with lesions in the subcoerulear nucleus display a remarkable behavior when they enter REM sleep. Rather than stretching out in the atonia that typically accompanies REM sleep, the cats he was studying stood up, looked around, and made movements of catching an imaginary mouse or running from an imaginary threat. Apparently if cats with damage to this brain region dream about catching mice or escaping from a threat, they are now acting out their dreams. We revisit Jouvet’s phenomenon in the next section, describing sleep disorders.
13-5 REVIEW
Neural Bases of Sleep
Before you continue, check your understanding.
Question 1
The ____________ in the central region of the brainstem is responsible for producing ____________ sleep.
Question 2
Loss of the RAS produces ____________.
Question 3
The peribrachial area and the MPRF, through activating pathways to the neocortex and spinal cord, are responsible for producing events associated with ____________.
Question 4
Cats with lesions to the ____________ nucleus act out their dreams.
Question 5
If you nod off to sleep at an inconvenient time, why does moving awaken you?
Answers appear in the Self Test section of the book.