Hydrogen bonds may form within or between molecules with polar covalent bonds
In liquid water, the negatively charged oxygen (δ–) atom of one water molecule is attracted to the positively charged hydrogen (δ+) atoms of another water molecule (Figure 2.10A). The bond resulting from this attraction is called a hydrogen bond. Later in this chapter you’ll see how hydrogen bonding between water molecules contributes to many of the properties that make water so important for living systems. Hydrogen bonds are not restricted to water molecules. Such a bond can also form between a strongly electronegative atom in one molecule and a hydrogen atom that is involved in a polar covalent bond in another molecule, or another part of the same molecule (Figure 2.10B).
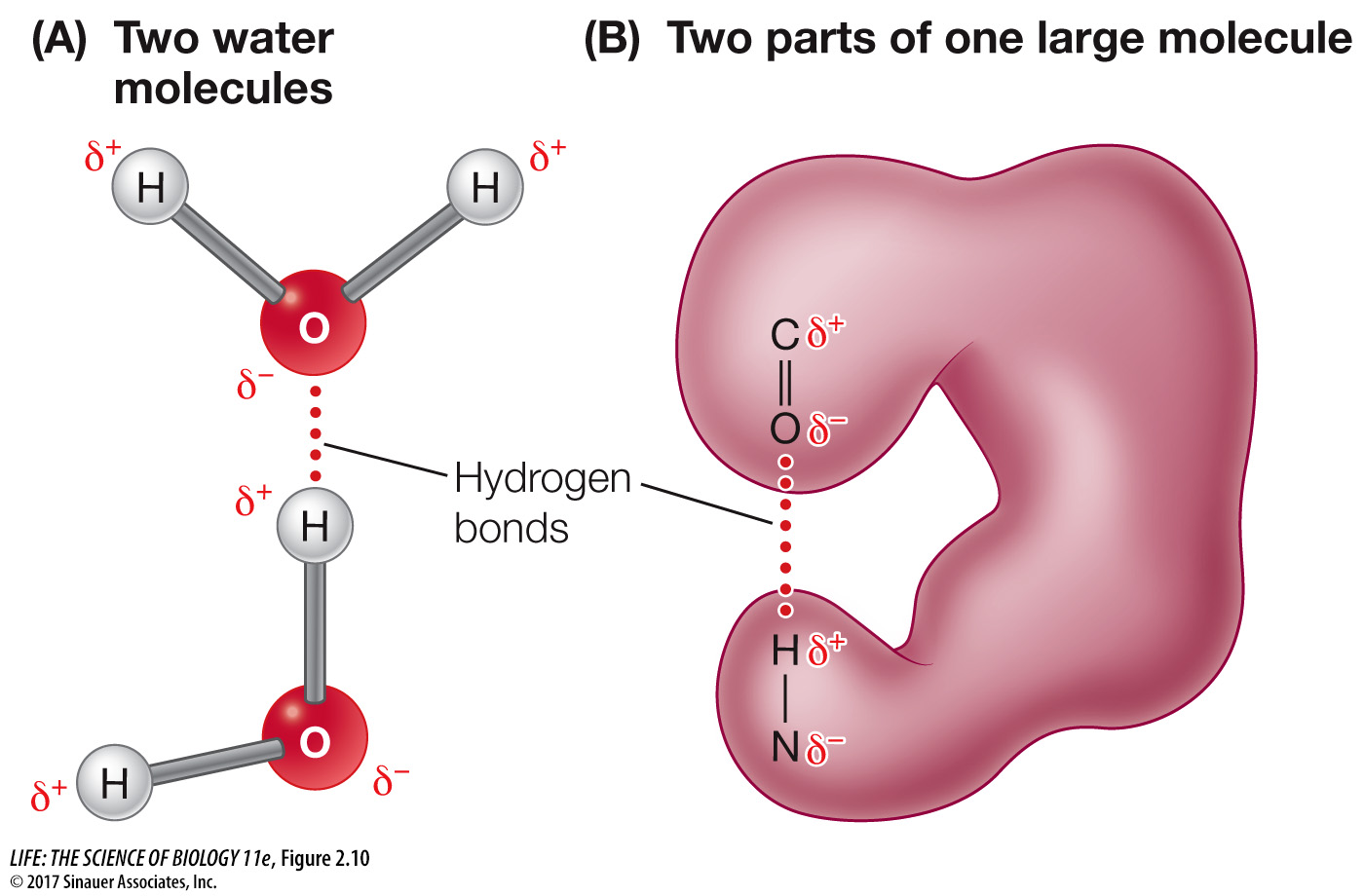
Q: What happens to a large molecule if increasing heat is applied? Which bonds or interactions are affected?
Heat will break hydrogen bonds. Because these bonds affect the interactions of some chemical groups with other groups at different locations in the molecule, the three-
A hydrogen bond is weaker than most ionic attractions because its formation is due to partial charges (δ+ and δ–). It is much weaker than a covalent bond between a hydrogen atom and an oxygen atom (see Table 2.1). Although individual hydrogen bonds are weak, there can be many of them within a single molecule or between two molecules. In these cases, the hydrogen bonds together have considerable strength and can greatly influence the structure and properties of substances. For example, hydrogen bonds play important roles in determining and maintaining the three-