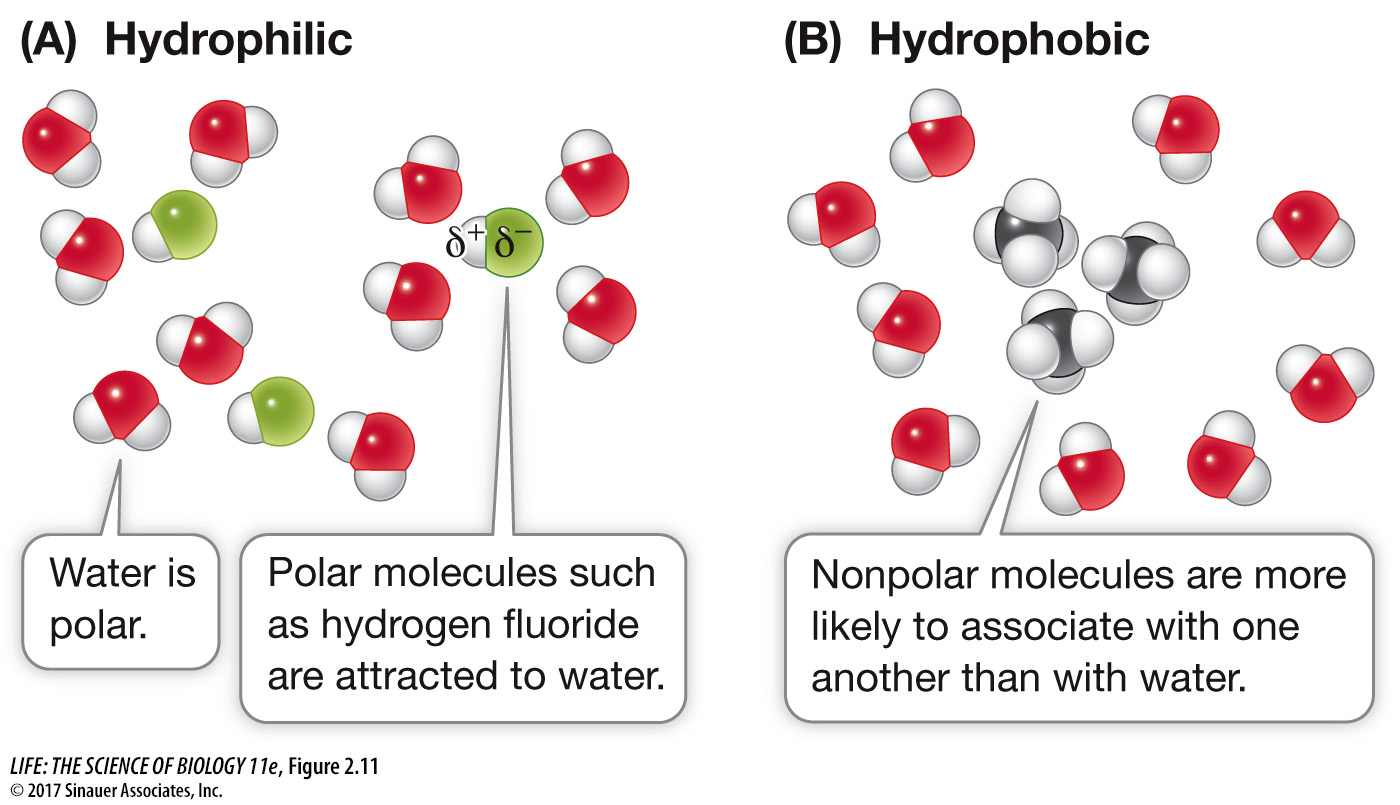
Just as water molecules can interact with one another through hydrogen bonds, any molecule that is polar can interact with other polar molecules through the weak (δ+ to δ–) attractions of hydrogen bonds. If a polar molecule interacts with water in this way, it is called hydrophilic (“water-loving”) (Figure 2.11A).
Nonpolar molecules, in contrast, tend to interact with other nonpolar molecules. For example, carbon (electronegativity 2.5) forms nonpolar bonds with hydrogen (electronegativity 2.1), and molecules containing only hydrogen and carbon atoms—called hydrocarbon molecules—are nonpolar. In water these molecules tend to aggregate with one another rather than with the polar water molecules. Therefore nonpolar molecules are known as hydrophobic (“water-hating”), and the interactions between them are called hydrophobic interactions (Figure 2.11B). Of course, hydrophobic substances do not really “hate” water; they can form weak interactions with it, since the electronegativities of carbon and hydrogen are not exactly the same. But these interactions are far weaker than the hydrogen bonds between the water molecules (see Table 2.1), so the nonpolar substances tend to aggregate.