key concept
2.2
Atoms Bond to Form Molecules
key concept
2.2
Atoms Bond to Form Molecules
A chemical bond is an attractive force that links two atoms together in a molecule. There are several kinds of chemical bonds (Table 2.1). In this section we will begin with covalent bonds, the strong bonds that result from the sharing of electrons. Next we will examine ionic attractions, which form when an atom gains or loses one or more electrons to achieve stability. We will then consider other, weaker kinds of interactions, including hydrogen bonds.
| Name | Basis of interaction | Structure | Bond energya |
|---|---|---|---|
| Covalent bond | Sharing of electron pairs |
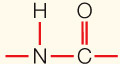
|
50– |
| Ionic attraction | Attraction of opposite charges |
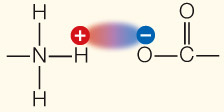
|
3– |
| Hydrogen bond | Electrical attraction between a covalently bonded H atom and an electronegative atom |
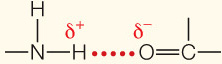
|
3– |
| Hydrophobic interaction | Interaction of nonpolar substances in the presence of polar substances (especially water) |
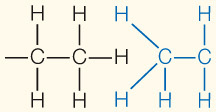
|
1– |
| van der Waals interaction | Interaction of electrons of nonpolar substances |
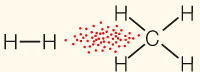
|
1 |
|
aBond energy is the amount of energy in kcal/mol needed to separate two bonded or interacting atoms under physiological conditions. |
|||
Animation 2.1 Chemical Bond Formation
focus your learning
Covalent bonds are very stable and break only with a large input of energy.
Polar covalent bonds occur when two atoms share bonding electrons in an unequal fashion, whereas nonpolar covalent bonds occur when the bonding electrons are shared equally.
Hydrophilic interactions occur between polar molecules, and hydrophobic interactions occur between nonpolar molecules.
van der Waals forces are weak, noncovalent attractive forces that occur between any two atoms.