Fats and oils are triglycerides
Chemically, fats and oils are triglycerides, also known as simple lipids. Triglycerides that are solid at room temperature (around 20°C) are called fats; those that are liquid at room temperature are called oils. Triglycerides are composed of two types of building blocks: fatty acids and glycerol. Glycerol is a small molecule with three hydroxyl (—OH) groups (thus it is an alcohol). A fatty acid is made up of a long nonpolar hydrocarbon chain and an acidic polar carboxyl group (—COOH). These chains are very hydrophobic because of their abundant C—
*connect the concepts As discussed in Key Concept 2.2, electronegativity is a measure of the attraction an atomic nucleus exerts on electrons in a covalent bond. When one interacting atom is much more electronegative than the other, a complete transfer of one or more electrons may take place.
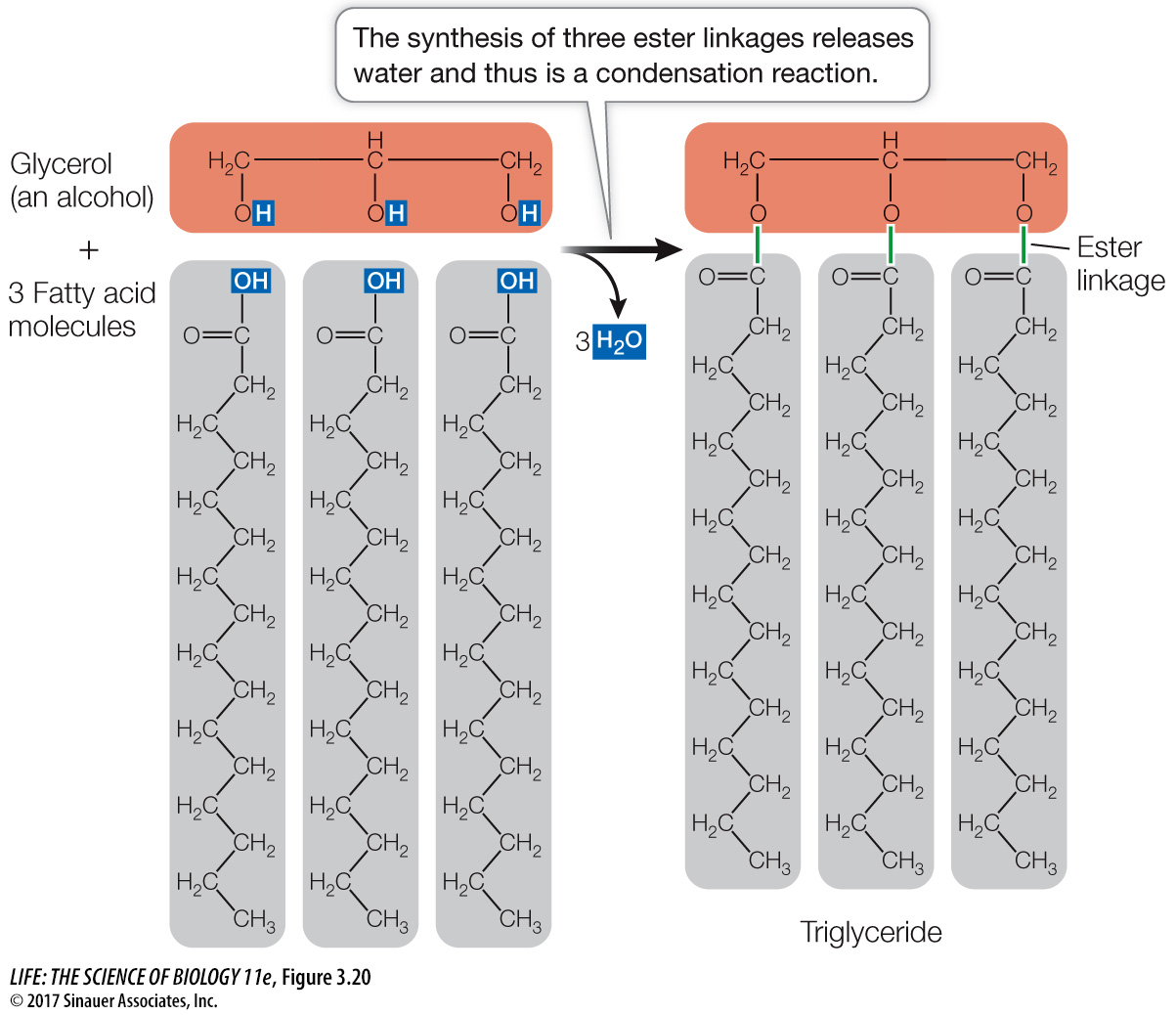
A triglyceride contains three fatty acid molecules and one molecule of glycerol. Making a triglyceride involves three condensation (dehydration) reactions. In each reaction, the carboxyl group of a fatty acid bonds with a hydroxyl group of glycerol, resulting in a covalent bond called an ester linkage and the release of a water molecule (Figure 3.20). The three fatty acids in a triglyceride molecule need not all have the same hydrocarbon chain length or structure; some may be saturated fatty acids, whereas others may be unsaturated:
In saturated fatty acids, all the bonds between the carbon atoms in the hydrocarbon chain are single bonds—
there are no double bonds. That is, all the bonds are saturated with hydrogen atoms (Figure 3.21A). These fatty acid molecules are relatively straight, and they pack together tightly, like pencils in a box. 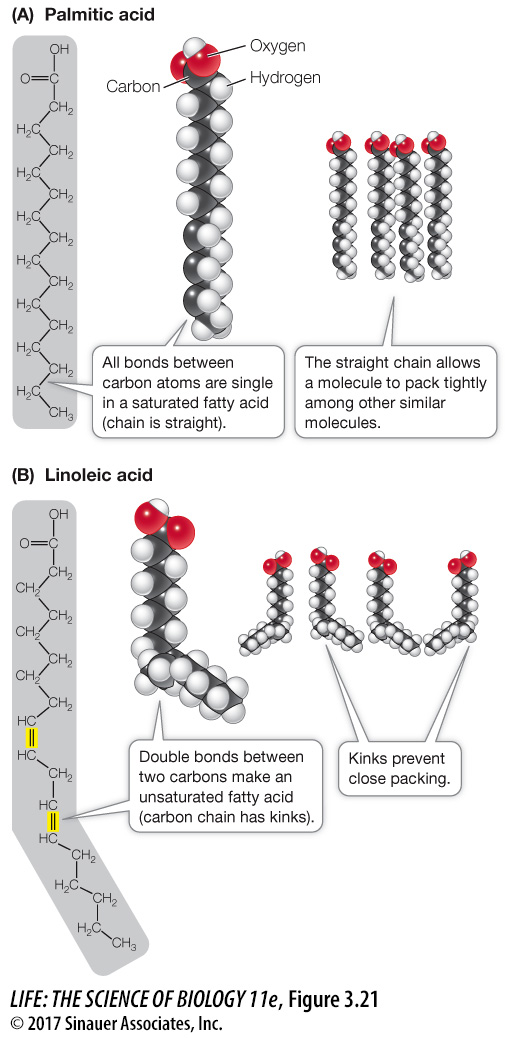 Figure 3.21 Saturated and Unsaturated Fatty Acids (A) The straight hydrocarbon chain of a saturated fatty acid allows the molecule to pack tightly with other, similar molecules. (B) In unsaturated fatty acids, kinks in the chain prevent close packing. The color convention in the models shown here (gray, H; red, O; black, C) is commonly used.
Figure 3.21 Saturated and Unsaturated Fatty Acids (A) The straight hydrocarbon chain of a saturated fatty acid allows the molecule to pack tightly with other, similar molecules. (B) In unsaturated fatty acids, kinks in the chain prevent close packing. The color convention in the models shown here (gray, H; red, O; black, C) is commonly used.In unsaturated fatty acids, the hydrocarbon chain contains one or more double bonds. Linoleic acid is an example of a polyunsaturated fatty acid that has two double bonds near the middle of the hydrocarbon chain, causing kinks in the molecule (Figure 3.21B). Such kinks prevent the unsaturated fat molecules from packing together tightly.
The kinks in fatty acid molecules are important in determining the fluidity and melting points of lipids. The triglycerides of animal fats tend to have many long-

Fatty acids are excellent storehouses for chemical energy. As you will see in Chapter 9, when the C—