Lipids form the hydrophobic core of the membrane
The lipids in biological membranes are usually *phospholipids. Recall from Key Concept 2.2 that some compounds are hydrophilic (“water-
Hydrophilic regions: The phosphate-
containing “head” of the phospholipid is electrically charged and therefore associates with polar water molecules. Hydrophobic regions: The long, nonpolar fatty acid “tails” of the phospholipid associate with other nonpolar materials; they do not dissolve in water or associate with hydrophilic substances.
*connect the concepts The straight chains of fatty acids allow phospholipids to pack closely together. Review the molecular structure and properties of phospholipids in Key Concept 3.4.
The chemical properties of phospholipids are such that when phospholipids coexist with water, they form a bilayer, with the fatty acid “tails” of the two layers interacting with each other and the polar “heads” facing the outside aqueous environment (Figure 6.2). The thickness of a biological membrane is about 8 nanometers (0.008 µm), which is twice the length of a typical phospholipid—
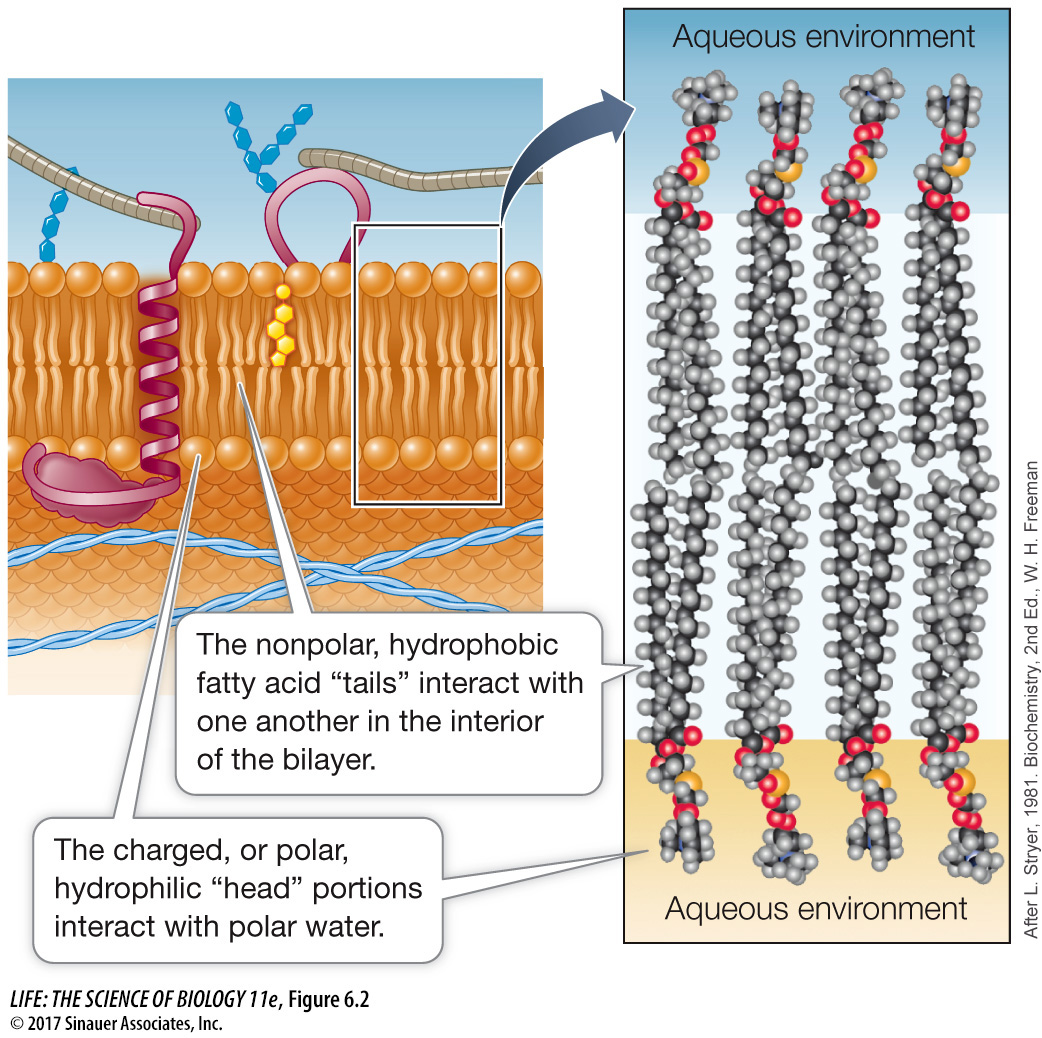
All biological membranes have a similar structure, but they differ in the kinds of proteins and lipids they contain. Membranes from different cells or organelles may differ greatly in their lipid composition. Phospholipids can differ in terms of fatty acid chain length (number of carbon atoms), degree of unsaturation (number of double bonds) in the fatty acids, and the polar groups present (see Chapter 3). The saturated chains allow close packing of fatty acids in the bilayer, whereas the “kinks” in unsaturated fatty acids (see Figure 3.21) make for a less dense, more fluid packing.
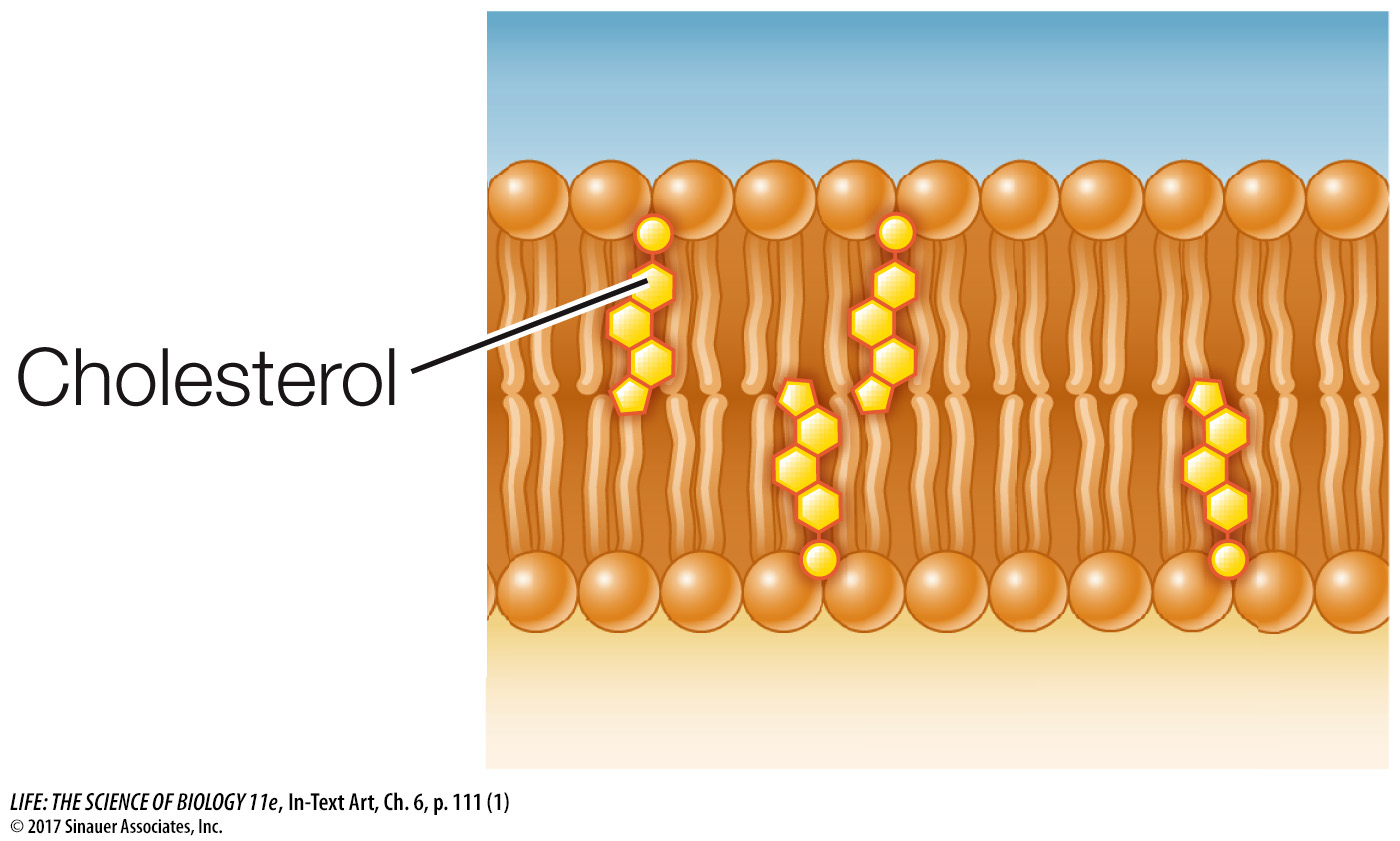
Up to 25 percent of the lipid content of an animal cell’s cell membrane may be the steroid cholesterol (see Key Concept 3.4). Cholesterol preferentially associates with saturated fatty acids. When present, cholesterol is important for membrane integrity; the cholesterol in your membranes is not hazardous to your health.
The fatty acids of the phospholipids make the hydrophobic interior of the membrane somewhat fluid—
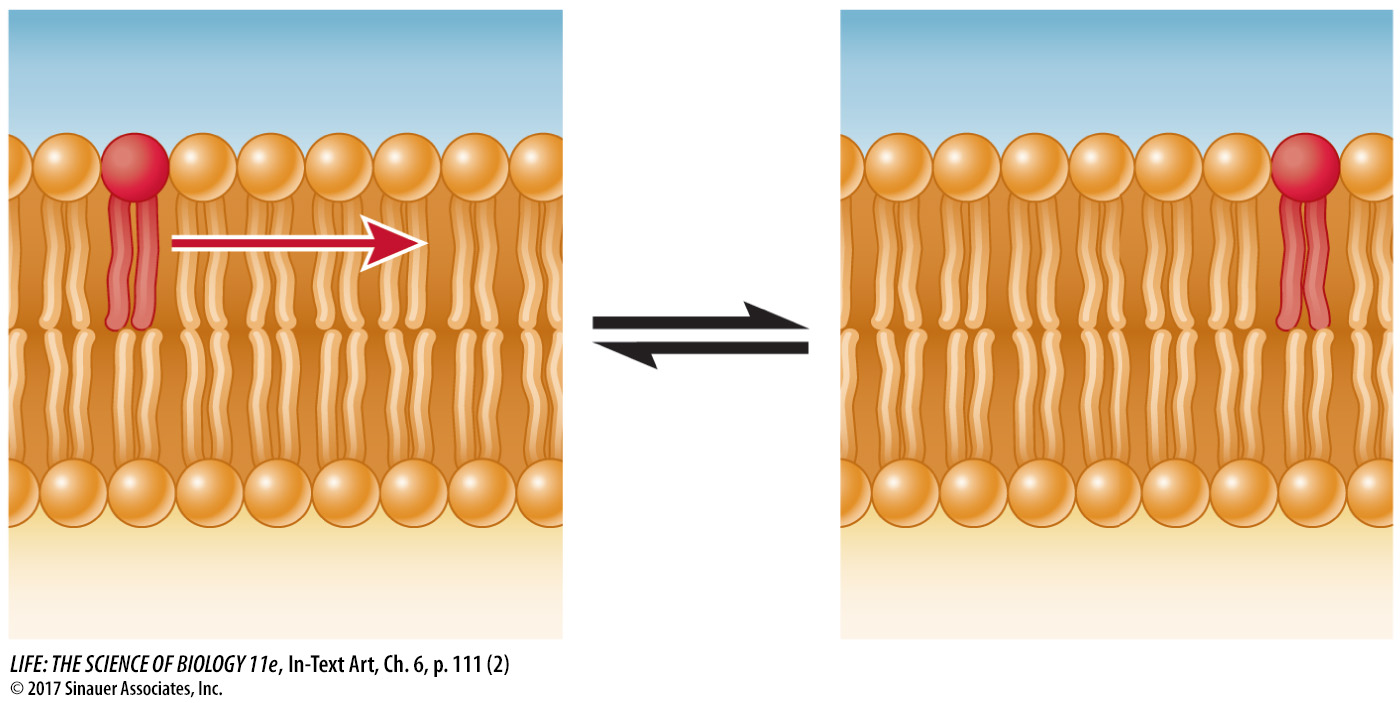
A given phospholipid molecule in the cell membrane can travel from one end of the cell to the other in a little more than a second! However, a phospholipid molecule in one half of the bilayer is unlikely to spontaneously flip over to the other side. For that to happen, the polar part of the molecule would have to move through the hydrophobic interior of the membrane. Since spontaneous phospholipid flip-
Membrane fluidity is affected by several factors, two of which are particularly important:
Lipid composition: Cholesterol and long-
chain, saturated fatty acids pack tightly beside one another, with little room for movement. This close packing results in less- fluid membranes. A membrane with shorter- chain fatty acids, unsaturated fatty acids, or less cholesterol is more fluid. Temperature: Because molecules move more slowly and fluidity decreases at reduced temperatures, cellular processes that take place within the membrane may slow down or stop under cold conditions in organisms that cannot keep their bodies warm. To address this problem, in some organisms the lipid composition of their membranes changes when they get cold, replacing saturated with unsaturated fatty acids and using fatty acids with shorter tails. These changes play a role in the survival of plants, bacteria, and hibernating animals during the winter.
Activity 6.2 Lipid Bilayer Composition Simulation