Enzymes can be regulated by inhibitors
Various chemical inhibitors can bind to enzymes, slowing down the rates of the reactions they catalyze. Some inhibitors occur naturally in cells; others are artificial. Naturally occurring inhibitors regulate metabolism; artificial ones can be used to treat disease, to kill pests, or to study how enzymes work. In some cases the inhibitor binds the enzyme irreversibly, and the enzyme becomes permanently inactivated. In other cases the inhibitor has reversible effects; it can separate from the enzyme, allowing the enzyme to function fully as before. The removal of a natural reversible inhibitor increases an enzyme’s rate of catalysis.
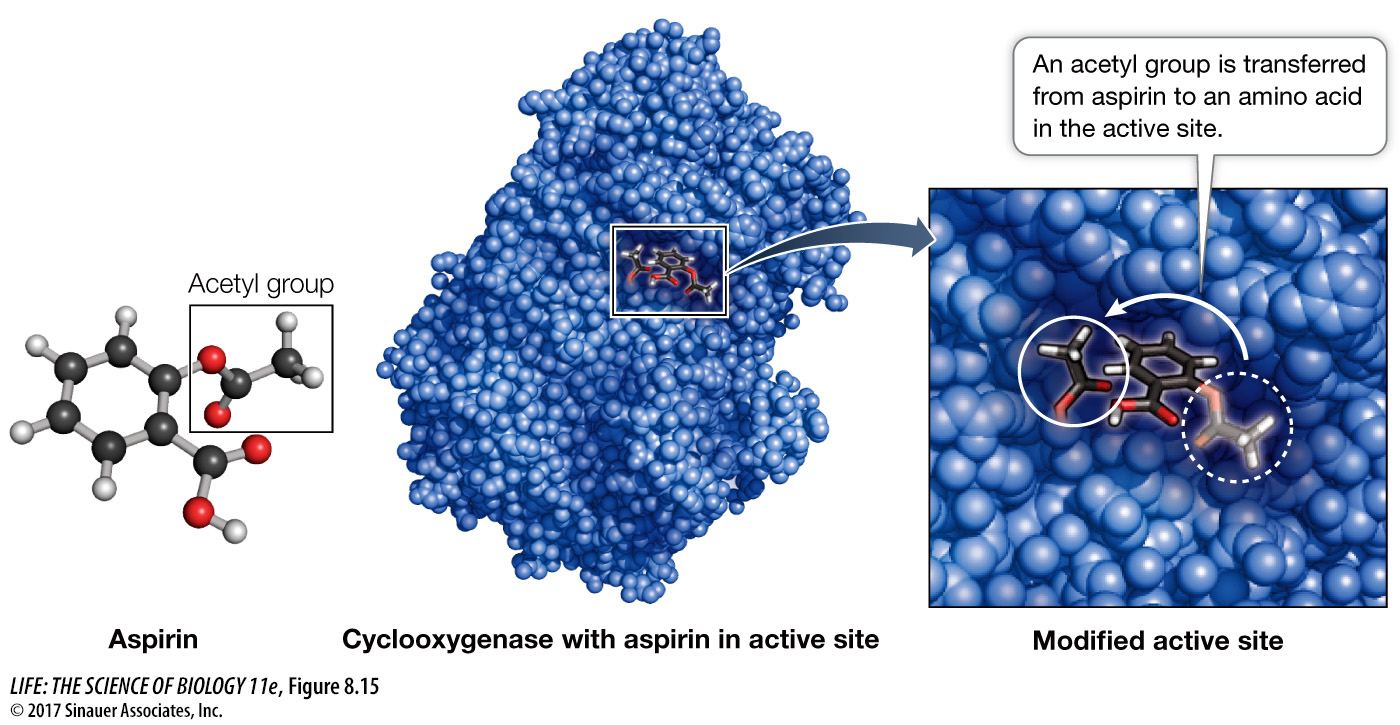
IRREVERSIBLE INHIBITION If an inhibitor covalently binds to certain side chains at the active site of an enzyme, it will permanently inactivate the enzyme by destroying its capacity to interact with its normal substrate. An example of an irreversible inhibitor is aspirin, which was described in the opening of this chapter. Aspirin (acetylsalicylic acid) binds to a target enzyme called cyclooxygenase (COX) near its active site. When it binds, aspirin releases its acetyl group (—CH2CH3) and this group of atoms is transferred to bond covalently to serine at its polar hydroxyl group (Figure 8.15). The group of atoms now “coating” serine sticks out into a channel in the protein and prevents the substrate, arachidonic acid, from reaching the active site. So prostaglandin is no longer produced and its stimulation of inflammation and pain are blocked. The elucidation of the mechanism of aspirin’s action as an inhibitor of a pathway involved in inflammation was a landmark in modern pharmacology, the study of drugs (Investigating Life: How Do Anti-Inflammatory Drugs Work as Enzyme Inhibitors?).
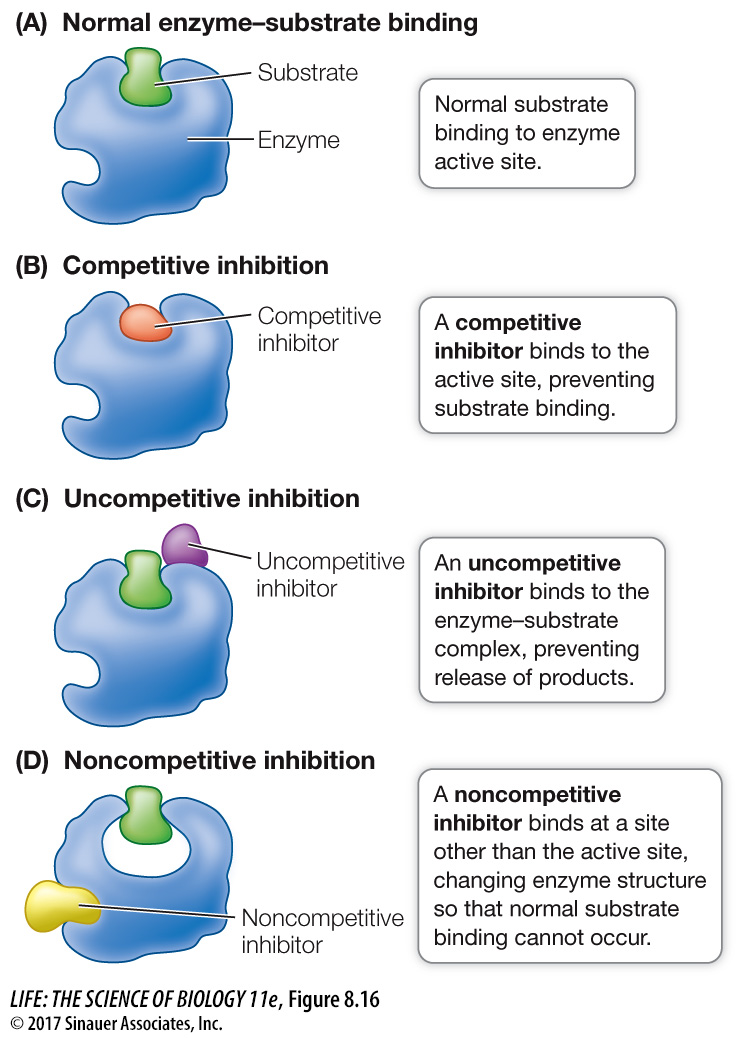
REVERSIBLE INHIBITION In some cases an inhibitor is similar enough to a particular enzyme’s natural substrate to bind noncovalently to its active site, yet different enough that the enzyme catalyzes no chemical reaction. While such a molecule is bound to the enzyme, the natural substrate cannot enter the active site and the enzyme is unable to function. Such a molecule is called a competitive inhibitor because it competes with the natural substrate for the active site (Figure 8.16A and B). In this case, the degree of inhibition depends on the relative concentrations of the substrate and the inhibitor: if the inhibitor concentration is higher, it is more likely to bind the active site of the enzyme than the substrate, and vice versa. The inhibition is reversible because if the concentration of substrate is increased or if the concentration of inhibitor is reduced, the substrate is more likely to bind, and the enzyme is active again.
Animation 8.1 Enzyme Catalysis
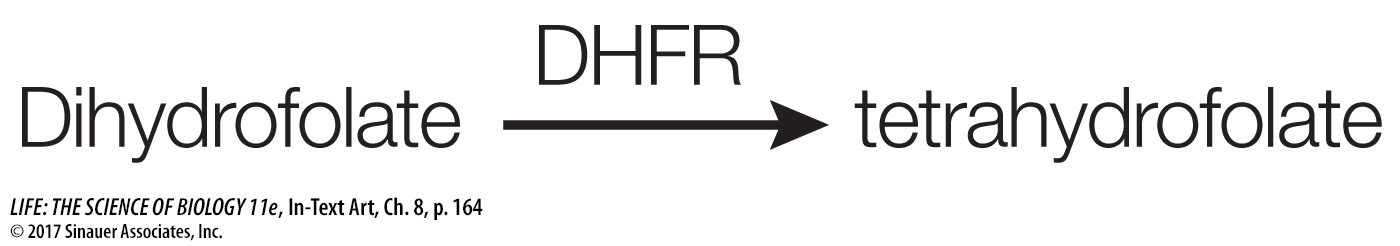
An example of a competitive inhibitor is the drug methotrexate. An important coenzyme in the formation of purines (components of nucleic acids) is tetrahydrofolate, which is formed from dihydrofolate in a reaction catalyzed by dihydrofolate reductase (DHFR):
When cancer cells reproduce, they need to replicate their DNA, and so they need to produce purines. This makes DHFR an ideal target for an anticancer drug. A team led by Sidney Farber at Harvard Medical School first showed that an analog of dihydrofolate could treat leukemia. The drug used by Farber (aminopterin) has since been replaced by a similar analog, methotrexate:
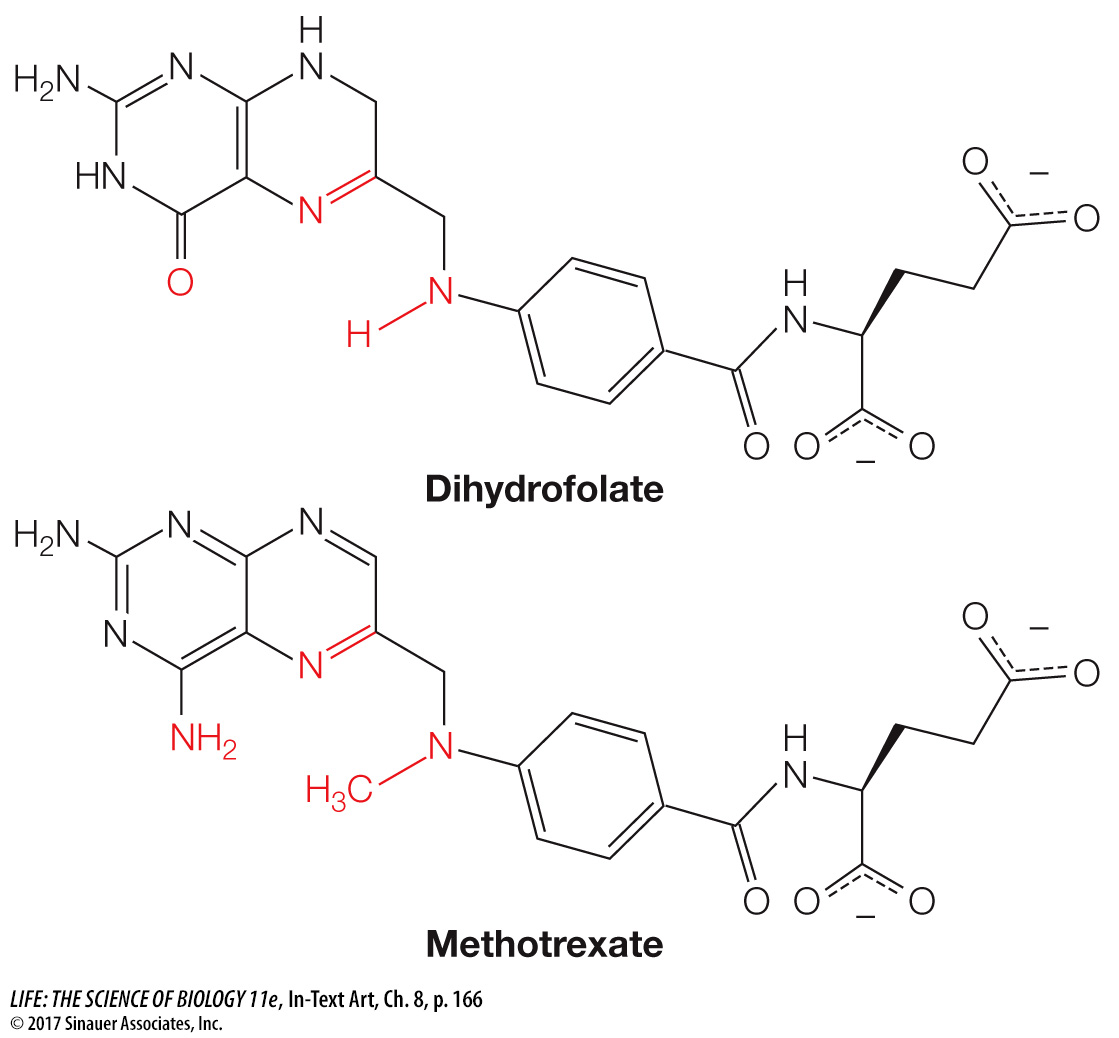
This successful drug is used to treat inflammatory diseases such as psoriasis and rheumatoid arthritis, as well as cancer.
investigating life
How Do Anti-
experiment
Original Papers: Vane, J. R. 1971. Inhibition of prostaglandin synthesis as a mechanism of action of aspirin-
Smith, J. B. and A. L Wells. 1971. Aspirin selectively inhibits prostaglandin production in human platelets. Nature 231: 235–
The opening story in this chapter describes how willow bark, the source of what became aspirin, is a centuries-
work with the data
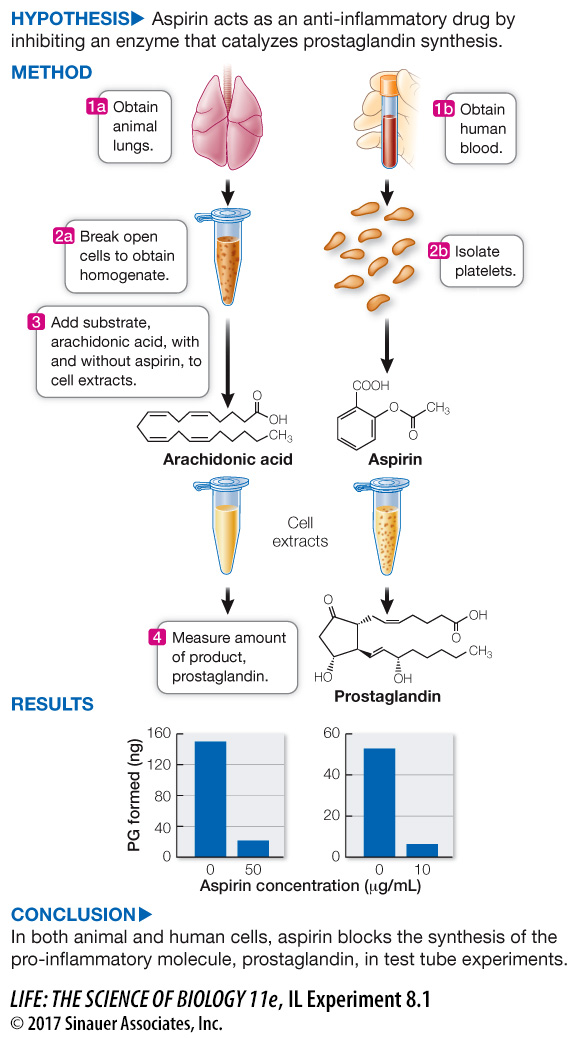
For his discovery of the mechanism by which aspirin relieves pain, John Vane was awarded the Nobel Prize and knighted by Queen Elizabeth II. Key to the experiments was the assumption that enzyme activity and mechanism are the same outside the organism as they are inside. In the lab, if given its substrate and the same set of environmental conditions as in the cytoplasm, an enzyme will catalyze production of its product.
QUESTIONS
1.
In the first set of experiments, lung tissue from guinea pigs was broken up to form a cell-
2.
A similar set of experiments was performed on human platelets (membrane-
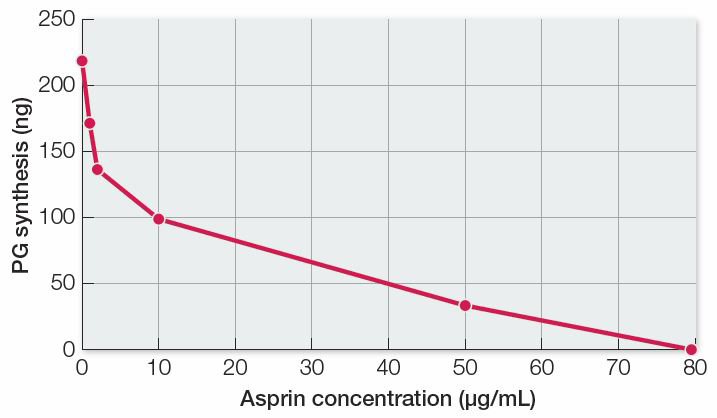
There is an inverse relationship between enzyme activity (PG synthesis) and aspirin concentration, so aspirin is an inhibitor of the reaction in lung tissue homogenates. Because aspirin also inhibited PG synthesis in human platelets, we can conclude that inhibition occurs in different tissues of different organisms.
| Aspirin concentration (µg/mL) | PG synthesis (ng) |
|---|---|
| 0 | 220 |
| 1 | 172 |
| 2 | 136 |
| 10 | 99 |
| 50 | 33 |
| 80 | 0 |
| Aspirin concentration (µg/mL) | PG synthesis (ng) |
|---|---|
| 0 | 53 |
| 0.01 | 48 |
| 0.1 | 35 |
| 1 | 18 |
| 10 | 7 |
3.
In a third set of experiments, platelets were isolated from human volunteers and their ability to make PG was measured (no aspirin present). Then these people were given a clinically effective dose of aspirin, and after a short period their platelets were isolated and PG synthesis measured (again, no aspirin in the test tube experiments). The results from three people are shown in Table C. How do these data either reinforce or refute your conclusion from Questions 1 and 2?
In all three individuals, aspirin given in vivo inhibited PG synthesis by platelets. This generalizes the results from the test tube experiment to a living organism.
| Prostaglandin synthesis (ng) | ||
|---|---|---|
| Individual | Before aspirin | After aspirin |
| 1 | 160 | 16 |
| 2 | 108 | 5 |
| 3 | 103 | 20 |
A similar work with the data exercise may be assigned in LaunchPad.
An uncompetitive inhibitor (Figure 8.16C) binds to the enzyme–
A noncompetitive inhibitor binds to an unbound enzyme at a site distinct from the active site. Binding causes a change in the shape of the enzyme that alters its activity (Figure 8.16D). The active site may no longer bind the substrate, or if it does, the rate of product formation may be reduced. Like competitive inhibitors, noncompetitive inhibitors can become unbound, so their effects are reversible.