Gene therapy offers the hope of specific treatments
If a cell lacks an allele that encodes a functional product, an optimal treatment would be to provide a functional allele. The objective of gene therapy is to add a new gene that will be expressed in appropriate cells in a patient. What cells should be targeted? There are two approaches:
Germ line gene therapy: The new gene is inserted into a gamete (usually an egg) or the fertilized egg. In this case, all cells of the adult will carry the new gene. Ethical considerations preclude the use of this method in humans.
Somatic cell gene therapy: The new gene is inserted into somatic cells involved in the disease. This method is being tried for numerous diseases, ranging from inherited genetic disorders to cancer.
There are two approaches to somatic cell gene therapy:
Ex vivo gene therapy: Target cells are removed from the patient, given the new gene, and then reinserted into the patient. This approach is being used, for example, for diseases caused by defects in genes that are expressed in white blood cells.
In vivo gene therapy: The gene is actually inserted directly into a patient, targeted to the appropriate cells. An example is a treatment for lung cancer in which a solution with a therapeutic gene is squirted onto a tumor.
Armed with knowledge of how genes are expressed (see Chapter 14) and regulated (see Chapter 16), physicians can design a therapeutic gene that contains not only a normal protein-
A major challenge has been getting the therapeutic gene into cells. Uptake of DNA into eukaryotic cells is a rare event, and once the DNA is inside a cell, its entry into the nucleus and expression are rarer still. One solution to these problems is to insert the gene into a carrier virus (a viral vector) that can infect human cells but has been altered genetically to prevent viral replication. An example is the DNA virus called adeno-
Adeno-
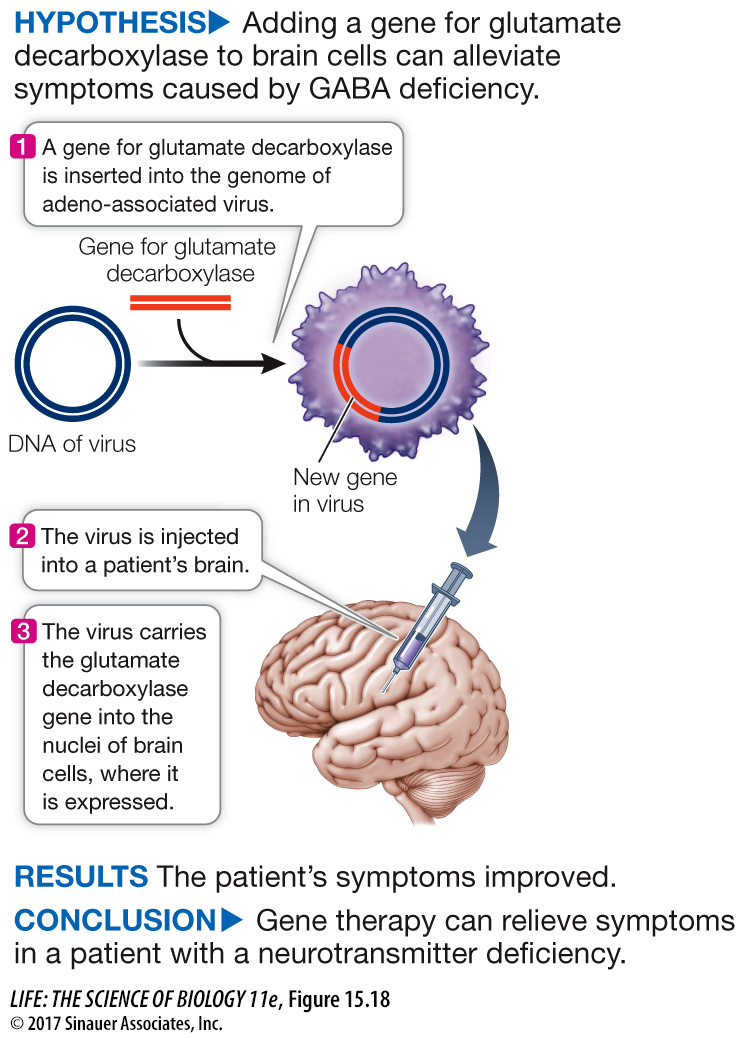
experiment
Figure 15.18 Gene Therapy
Original Paper: LeWitt, P. A. et al. 2011. AAV2-
Andrew Feigin and his colleagues showed that a virus can be used to insert a therapeutic gene into the brains of patients with Parkinson’s disease.
A work with the data exercise that accompanies this figure may be assigned in LaunchPad.