Antibody protein structure reflects function
Antibodies belong to a class of proteins called immunoglobulins. There are several types of immunoglobulins, but all contain a tetramer consisting of four polypeptide chains: two “heavy chains” and two “light chains” (Figure 41.8). In each immunoglobulin molecule, the two light chains are identical, and the two heavy chains are identical. Disulfide bonds hold the chains together, characteristically in a Y-like shape.
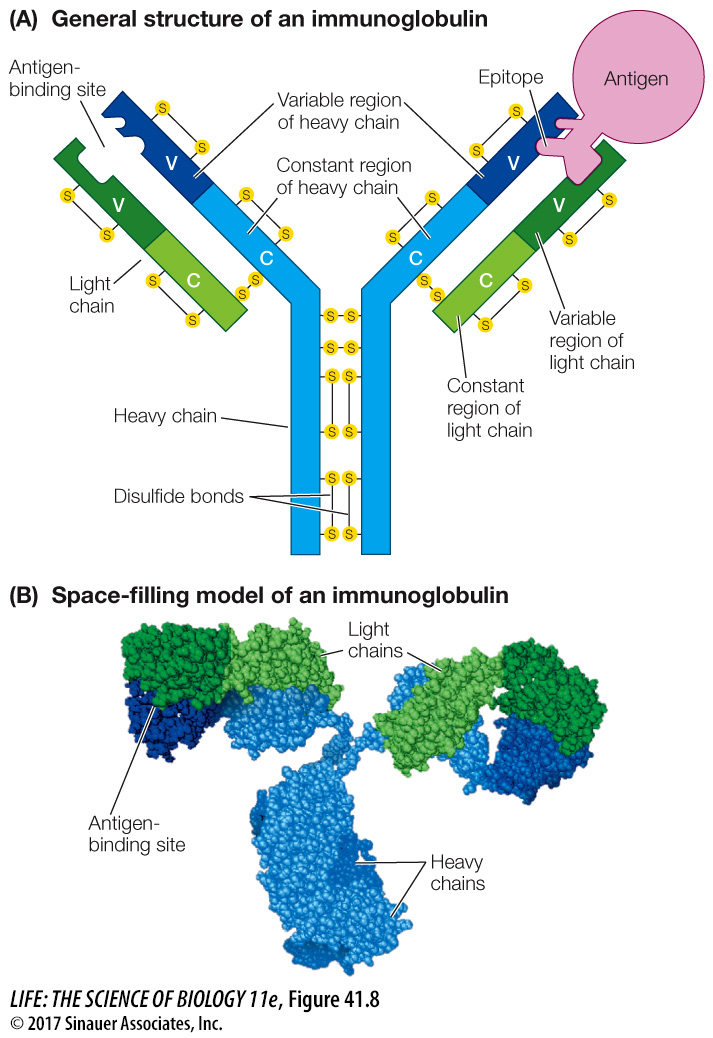
Figure 41.8 The Structure of an Immunoglobulin Four polypeptide chains (two light, two heavy) make up an immunoglobulin molecule. Both diagrammatic (A) and space-filling (B) representations of immunoglobulin are shown here.
Activity 41.4 Immunoglobulin Structure
Each of the four polypeptide chains has a constant region and a variable region (designated C and V in Figure 41.8A):
The amino acid sequences of the constant regions are similar among the immunoglobulins. They determine the destination and function—the class—of each immunoglobulin.
The amino acid sequences of the variable regions are different for each specific immunoglobulin. Their three-dimensional antigen-binding sites are determined by their secondary structures and are responsible for antibody specificity.
Antigen–antibody binding is noncovalent. The attractive forces involved in holding antigen and antibody together include hydrogen bonding, ionic attractions, hydrophobic interactions, and van der Waals forces (see Key Concept 2.2). These attractive forces are generated from particular amino acids at the binding site on both molecules. So for an antibody protein, the sequence of amino acids at the binding site is crucial to its specificity. Generating antibody diversity is thus a molecular genetics challenge, as we will soon discuss.
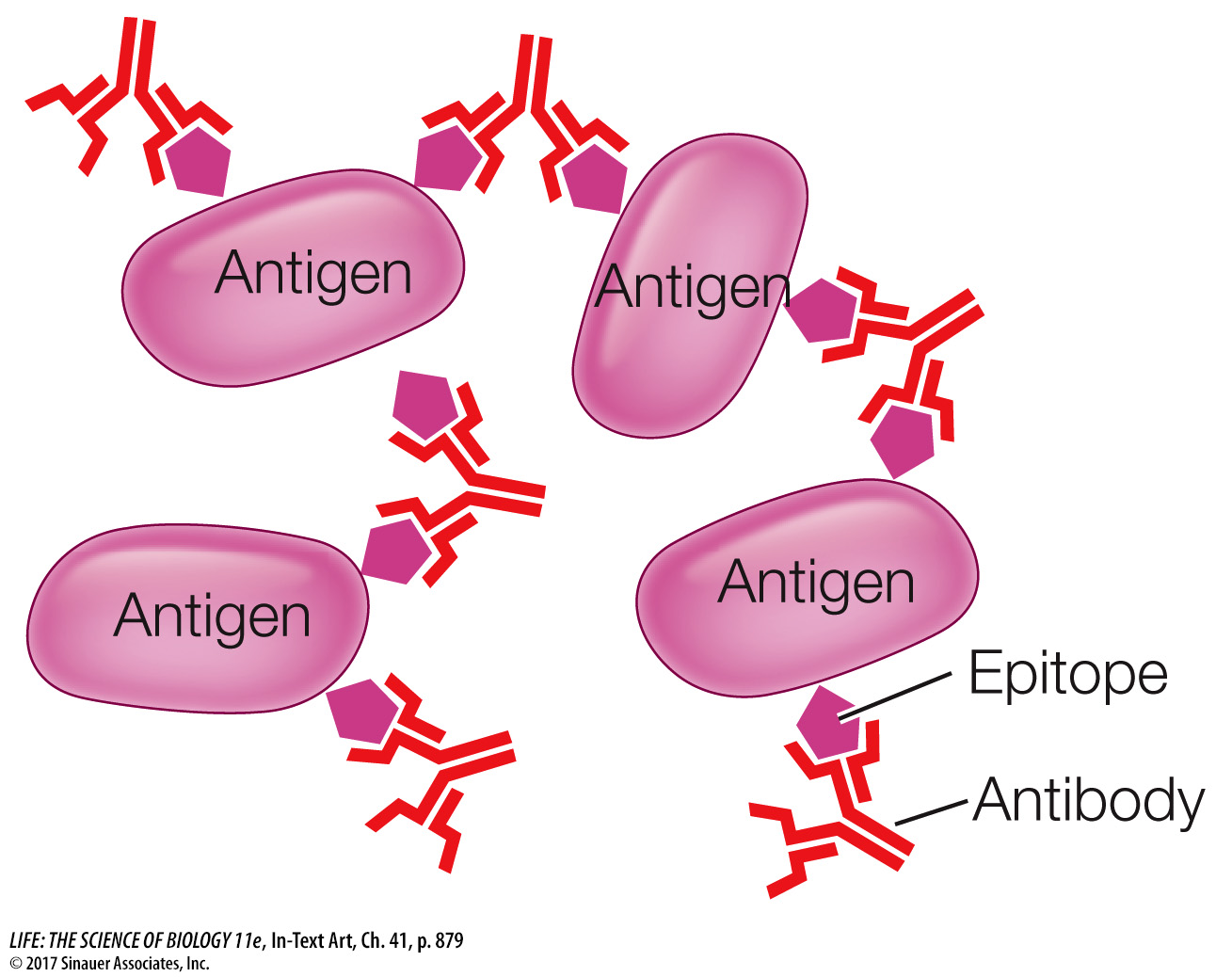
The two antigen-binding sites on each immunoglobulin molecule are identical, making the antibody bivalent (bi, “two,” + valent, “binding”), meaning the molecule can bind two antigen molecules at once. Bivalency, in addition to the presence of multiple epitopes on the surfaces of many antigens (including large proteins, viruses, and bacteria, as you saw in the case of the influenza virus explored in Investigating Life: What Are the Mechanisms and Implications of Long-Lasting Immunity?), permits antibodies to form large complexes with the antigens. These complexes are easy targets for ingestion and breakdown by phagocytes.