Energy needs and expenditures can be measured
Energy—
Just about any food container you pick up in the United States carries the label “Nutrition Facts,” which includes the item “Calories.” How do Calories (or kcal) relate to the discussion in Chapter 9 about how energy in the chemical bonds of food molecules is transferred to the high-
The reason is found in the *laws of thermodynamics, which tell us that energy cannot be created or destroyed, but can be converted from one form to another.
*connect the concepts The laws of thermodynamics and their application to biological energetics are described in Key Concept 8.1.
However, every energy conversion is inefficient; a large portion of the original energy always ends up as heat. Whether we are using the energy in glucose to make ATP or are using that ATP to power muscle contraction or ion transport, most of the available chemical energy is lost as heat. The bottom line is that if an animal is not growing, not doing any external work, and not changing its body temperature, the heat it loses to the environment is a measure of its total energy expenditure, or metabolism.
An animal’s energy needs must be met by the ingestion, digestion, and assimilation of food. The basal energy expenditure (basal metabolic rate, BMR) of a human is 1,300–
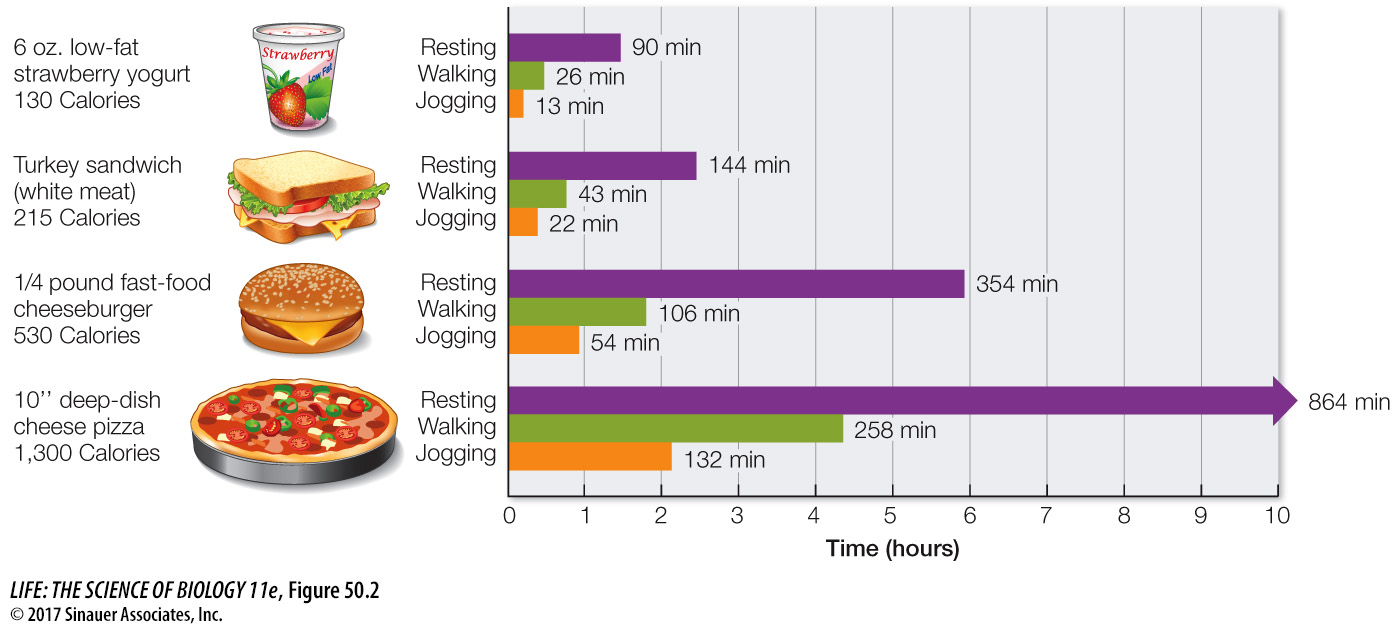
The components of food that provide energy are fats, carbohydrates, and proteins. Fats yield 9.5 Cal/gram, carbohydrates 4.2 Cal/gram, and proteins about 4.1 Cal/gram. Figure 50.2 shows some equivalencies of food, energy, and energy consumption.
Even though the units calorie, kilocalorie, and Calorie remain in popular use, most scientists now use the International System of Units (ISU). In this system the basic unit of energy is the joule: 1 joule = 0.239 calories, and the measure of energy use is 1 joule/second = 1 watt. You are familiar with light bulb ratings, so think about that when you convert kcal/day into watts. The 1,700 Cal/day energy expenditure of the average man converts to 82 watts (note that a watt includes the time dimension, so it is a rate of energy use).
Thus it is possible to quantify the caloric value of any food an animal eats. It is also possible to quantify the caloric expenditure of any activity or behavior an animal performs. By comparing calories consumed with calories expended, we can construct energy budgets that allow ecologists and evolutionary biologists to apply a *cost–
*connect the concepts Any behavior involves energetic costs, opportunity costs, and risk costs and can be measured in terms of its effect on reproductive success. Key Concept 52.4 explores costs and benefits of various behaviors, illustrating how measures of metabolism permit quantitative analysis of evolutionary adaptations.