LESSON 71: Some Things Never Change: Conservation of Mass
THINK ABOUT IT
During chemical and physical changes, the atoms in a substance rearrange. Sometimes, a solid forms or disappears. Sometimes, the substances change appearance or substances with new properties appear. But is matter really “appearing” or “disappearing”?
How does mass change during a chemical or physical change?
To answer this question, you will examine
Tracking Mass
Conservation of Mass
Tracking Mass
EXPLORING THE TOPIC
Tracking Mass
In Unit 1: Alchemy, you learned that matter is conserved during chemical reactions. If matter is not created or destroyed during physical and chemical changes, then there should be no measurable difference in mass before and after a change. Three chemical procedures are described here. The mass before and after each change is shown on the balances.
PHYSICAL CHANGE—DISSOLVING A SOLID
Consider what happens when water is added to solid sodium carbonate.
Na2CO3(s) → Na2CO3(aq)
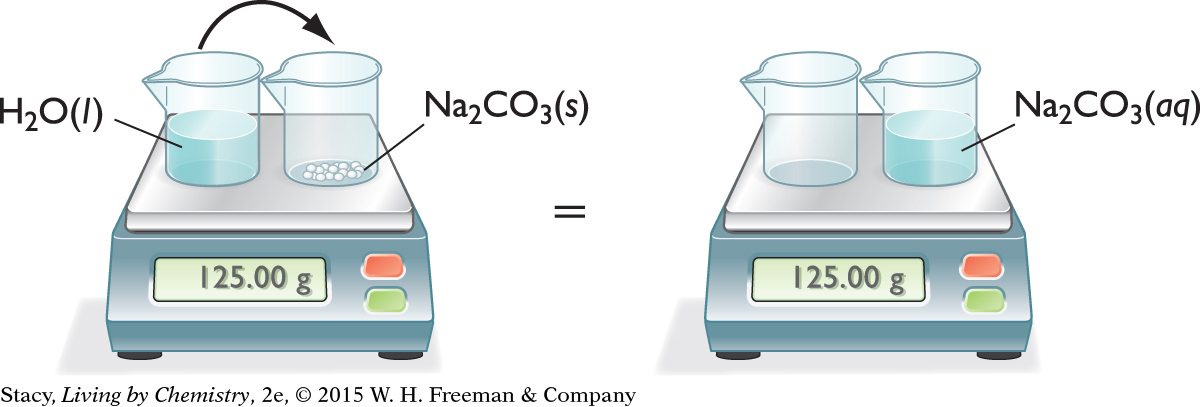
The solid seems to disappear into the liquid. However, notice that the total mass doesn’t change. It is the same before and after the substances are mixed. The ions in the white solid have spread evenly throughout the water.
This change can also be represented by the chemical equation:
Na2CO3(s) → 2Na+(aq) + CO32–(aq)
Even though this compound breaks apart into ions, the mass is the same before and after the change.
ENVIRONMENTAL CONNECTION
ENVIRONMENTAL
CONNECTION
In 2009, about three-quarters of the U.S. population had access to curbside recycling programs. About 42% of all paper, 40% of all plastic soft drink bottles, 55% of all aluminum cans, 57% of all steel packaging, and 52% of all major appliances are now recycled. The photo shows a paper-recycling plant.

CHEMICAL CHANGE—PRODUCING A SOLID
Consider a chemical change in which a new solid forms. Adding an aqueous solution of sodium chloride to an aqueous solution of silver nitrate produces aqueous sodium nitrate and solid silver chloride.
| NaCl(aq) | + | AgNO3(aq) | → | NaNO3(aq) | + | AgCl(s) |
| 58 g | + | 170 g | = | 85 g | + | 143 g |
Even though a solid is produced, the mass of the products is still equal to the mass of the reactants. No mass was gained or lost.
CHEMICAL CHANGE—PRODUCING A GAS
Now consider a chemical change that produces a gas as a product. Adding an aqueous solution of hydrochloric acid to solid magnesium metal produces an aqueous solution of magnesium chloride and bubbles of hydrogen gas.
2HCl(aq) + Mg(s) → H2(g) + MgCl2(aq)
Important to Know
It is difficult to measure the weight of a sample of gas, so it is tempting to conclude that gases do not have mass. However, as you saw in Unit 3: Weather, gases are made of molecules and they do have mass.
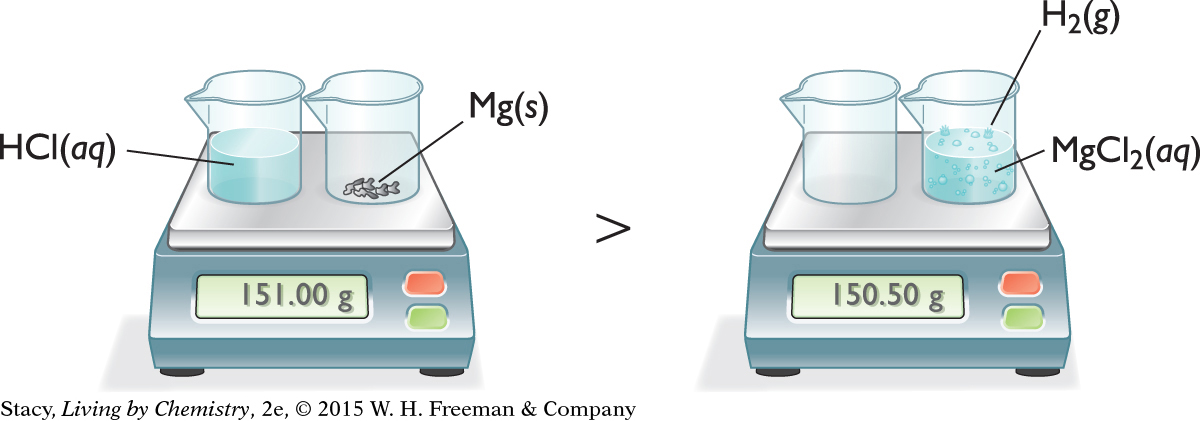
According to the balance, some mass appears to have been lost. However, notice that some gas escaped from the beaker. If you were able to trap the gas that escaped and measure its mass, it would account for the difference in masses seen here.
Example
Chemical Change—Decomposing Calcium Carbonate
Examine this description of a chemical change along with its chemical equation.
Verbal description: Solid calcium carbonate is heated to produce solid calcium oxide and carbon dioxide gas.
Chemical equation: CaCO3(s) → CaO(s) + CO2(g)
50 g = 28 g + ?
mass of reactants = mass of products
What would you expect the mass of the carbon dioxide gas to be? Explain your reasoning.
Solution
The mass of the products on the right side must equal the mass of the reactants on the left side. So, the mass of the carbon dioxide produced is 22 grams.
Conservation of Mass
Conservation of Mass
ENVIRONMENTAL CONNECTION
ENVIRONMENTAL
CONNECTION
Bacteria can break down organic waste such as food scraps and dead leaves. But if garbage is piled deep in a landfill, the bacteria can’t access the air and water they need to break things down. Some trash companies collect food scraps and garden waste separately, so they can be composted.

The fact that the mass of the reactants is equal to the mass of the products follows the law of conservation of mass. Mass is conserved during chemical and physical changes because no atoms are created or destroyed.
GETTING RID OF GARBAGE
People generate a lot of waste: cups, cans, bottles, candy wrappers, paper, and diapers. Most of this waste just keeps piling up in landfills. Some of the waste is quite toxic. In the United States alone, about 135 million tons of garbage are put into landfills each year. Will it be there forever?
Atoms are not created or destroyed; they are simply rearranged to form new substances. Therefore, everything on the planet is made up of a limited number of atoms. Even your body does not have the same atoms in it that it did several years ago. You are continually generating new cells of every kind and getting rid of the old ones.
The law of conservation of mass is important in considering waste disposal. You cannot get rid of the atoms in the waste that you throw away. Smelly, toxic garbage accumulates in large quantities in waste disposal sites. A small portion of it may biodegrade—that is, get broken down into harmless products by microorganisms—but the process can take decades. The waste will be there unless scientists find ways to convert more of our waste into useful products. Given this fact, it makes sense to reuse and recycle as much as possible.
LESSON SUMMARY
LESSON SUMMARY
ENVIRONMENTAL CONNECTION
ENVIRONMENTAL
CONNECTION
Compounds are sometimes recycled in surprising ways. For example, PETE plastic bottles can be recycled to make T-shirts, suits, and fleece!

How does mass change during a chemical or physical change?
When chemical and physical changes take place, the atoms in substances rearrange. A rearrangement may be a physical change, such as the mixing of two substances, or a chemical change, the formation of new compounds. However, the atoms involved in these rearrangements cannot be created or destroyed. A chemical equation tracks the atoms involved in chemical and physical changes. All of the atoms are accounted for, so the mass of the products is identical to the mass of the reactants. This is known as the law of conservation of mass.
Exercises
Reading Questions
Explain the law of conservation of mass.
Explain how the law of conservation of mass applies to garbage.
Reason and Apply
Below is a chemical equation along with a verbal description of the reaction.
Verbal description: Gaseous sulfur trioxide is added to liquid water to produce aqueous sulfuric acid.
Chemical equation: SO3(g) + H2O(l) → H2SO4(aq)
Was matter lost or gained during this reaction? Explain how you could prove this by taking measurements.
The left side of the equation shows one atom of S, two atoms of H, and four atoms of O. How many atoms of each element are on the right side of the equation? How does this provide evidence for the law of conservation of mass?
When an ice cube melts, which of these quantities will change?
The number of atoms it contains
Its mass
Its volume
All of the above
None of the above
Explain what happens to the number of atoms, the mass, and the weight of the water in a glass when it evaporates.
Write a paragraph to convince a friend that mass is conserved when chemical and physical changes take place. Give evidence.
What would you have to do to prove that matter is conserved when a piece of paper is burned?