LESSON 87: Watered Down: Dilution
THINK ABOUT IT
In Unit 1: Alchemy, a “golden” penny was made with the help of a strong base called sodium hydroxide. In Unit 2: Smells, you added concentrated sulfuric acid to a mixture of chemicals to make a sweet-smelling ester. Whenever you work with very concentrated acids and bases, you are instructed to rinse with large amounts of water if you get any on your skin or clothing. How does this help?
How does dilution affect acids and bases?
To answer this question, you will explore
Diluting Acidic Solutions
Approaching pH 7
Diluting Acidic Solutions
EXPLORING THE TOPIC
Diluting Acidic Solutions
If you spill concentrated acid on your skin, it will begin to sting and burn right away. If you spill it on your clothing, you will eventually see holes in the cloth. The best thing you can do for this situation is to put a lot of water on the part of your skin or clothing where the acid spilled.
The process of adding more and more water to a solution is called dilution. You could say that the solution is becoming more dilute or “watered down.” This changes the number density of the solute particles in solution.

The effect of diluting a concentrated solution can be demonstrated with a glass of grape juice. The color of the grape juice becomes paler as more water is added. And the more dilute the grape juice becomes, the weaker it tastes.
Acidic solutions are not necessarily colorful like grape juice, but the concept of dilution is the same: The solution becomes less concentrated. When you dilute an acid, you can track what is happening by testing the pH.
The illustration below shows a particle view of what happens when you dilute an acidic solution. A few drops of red dye have been added so that you can visually keep track of the different solutions. The solution is diluted with water in the beaker. The starting solution in the test tube has a pH of 2. The particle-view squares show how the H+ ions and Cl− ions end up farther apart as more water is added.
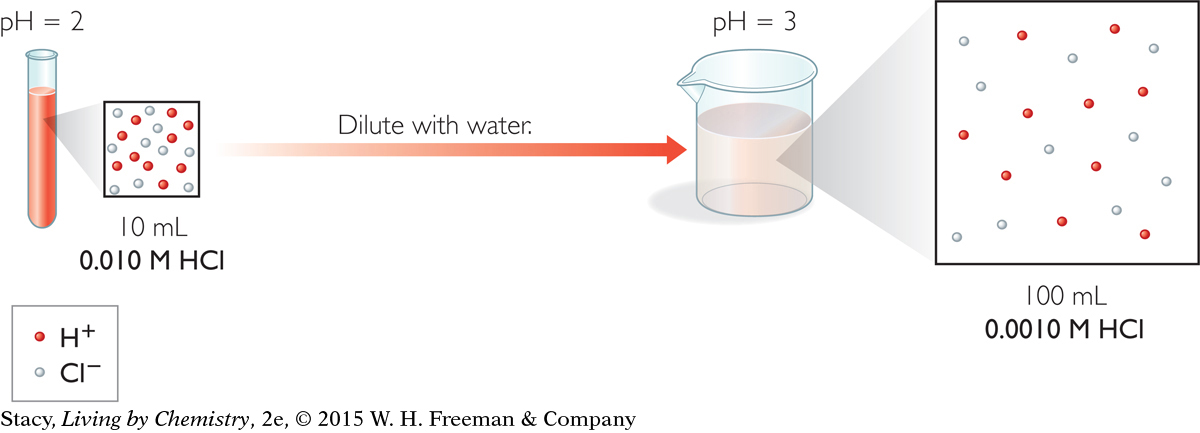
Dilution of a strong acid or base can make it safer. This is why you can swim in a swimming pool even though the water contains both acids and bases.
Example 1
Diluting HCl
Imagine you have a 1.0 mL water sample containing 0.10 M hydrochloric acid, HCl. You want to get it to a safe pH of 6. How much water do you need to add?
Solution
The pH of 0.10 M HCl is 1. The H+ concentration needs to change from 1.0 × 10−1 M to 1.0 × 10−6, a factor of 105, or 100,000. So you need to add 99,999 mL of water to 1 mL 0.10 M HCl to get it to a safe pH of 6.
Approaching pH 7
Approaching pH 7
RECREATION CONNECTION
RECREATION
CONNECTION
Swimming pools require a slightly basic pH, between 7.2 and 7.8. If the pH is too low, eye irritation occurs and metal pumps may corrode. If the pH is too high, the water may become cloudy and the chlorine may not work to combat algae. Sodium carbonate is added to pools to keep the pH in the right range. Indicators are used to test the pH.

Suppose you continue to dilute the 0.0010 M HCl solution in the large beaker in the previous illustration to a concentration of 0.00010 M HCl and pH of 4. If you continue to dilute, what is the lowest H+ concentration you can obtain? What is the lowest pH you can reach?
If you take 1 mL of 0.00010 M HCl and add 9 mL of water, the new solution will have a concentration of 0.000010 M HCl and a pH of 5. If you take 1 mL of 0.000010 M HCl and add 9 mL of water, the new solution will have a concentration of 0.0000010 M HCl and a pH of 6. You can continue this process, diluting the acid until the pH becomes 7. At this point, the liquid is no longer acidic. It is neutral.
As you dilute an acidic solution, its H+ concentration gets closer and closer to the H+ concentration of water, 1 × 10−7 moles per liter. However, the pH does not increase beyond 7, no matter how much you dilute the solution. In other words, the solution does not become basic. You cannot start with an acidic solution and dilute it to make a basic solution.
Likewise, you cannot dilute a basic solution to make an acidic solution. When you dilute a basic solution, the OH− concentration decreases, the H+ concentration increases, and the pH decreases toward 7.
Big Idea
Big Idea
An acid can never be made into a base by diluting with water. A base can never be made into an acid by diluting with water.
Example 2
Diluting NaOH
Imagine that you have 10 mL of a 0.010 M solution of sodium hydroxide, NaOH.
What is the H+ concentration of this solution?
What is the pH?
What is the NaOH concentration if you dilute the 0.010 M solution by adding 90 mL of water?
Does the pH increase or decrease after diluting the 0.010 M NaOH solution?
What is the pH of the solution after adding 90 mL of water?
Solution
The OH− concentration is 0.010 mole per liter, which is equivalent to 1 × 10−2 M. Because [H+] [OH−] = 1 × 10−14, the H+ concentration is 1 × 10−12 M. The exponents sum to −14.
pH = −log [H+] = −log 10−12 = 12
If you add 90 mL water to 10 mL of 0.010 M NaOH, then you have 100 mL. The volume has increased tenfold. So the concentration after diluting is 0.0010 M NaOH.
As the OH−concentration decreases, the H+ concentration increases. So the pH decreases when you dilute 0.010 M NaOH to 0.0010 M.
The OH− concentration is now 1.0 × 10−3 M. Because [H+] [OH−] = 1.0 × 10−14, [H+] = 1 × 10−11 M.
pH = −log [1 × 10−11] = 11
So the pH has decreased, from 12 to 11, as you might expect. Diluting with water makes a solution more neutral.
LESSON SUMMARY
LESSON SUMMARY
How does dilution affect acids and bases?
KEY TERM
dilution
When water is added to an acidic solution, the result is a “watering down” of the solution. The H+ concentration of the solution decreases, and the pH increases toward 7. When water is added to a concentrated basic solution, the OH− concentration decreases. This increases the H+ concentration and the pH decreases toward 7. Acids cannot be made into bases and bases cannot be made into acids by adding water.
Exercises
Reading Questions
When you add water to an acidic solution, describe what happens to the pH of that solution.
Explain why you cannot turn an acid into a base by diluting it with water.
Reason and Apply
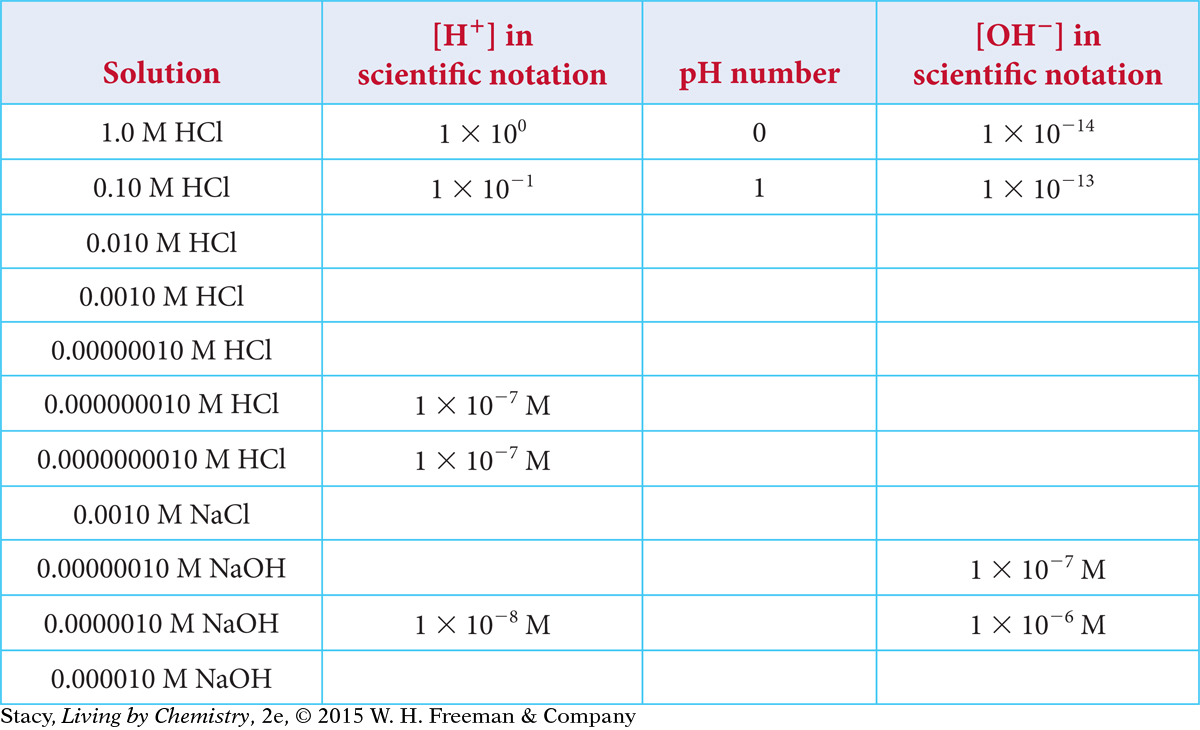
Copy this table and fill in the missing information.
How much water do you need to add to 10 mL of a solution of HCl with a pH of 3 to change the pH to 6?
Explain why the pH of a 1 × 10−9 M HCl solution is 7 even though the exponent is –9.
Explain why the pH of a NaCl solution does not change if you dilute the solution.
Imagine that you have 0.75 L of a 0.10 M HCl solution.
How many moles of H+ are in the 0.75 L?
If you add 0.35 L of water, what is the new concentration of the solution?
What is the pH after adding 0.35 L?
Imagine that you have 15 mL of a 0.025 M HCl solution.
How many moles of H+ are in the 15 mL?
If you add 25 mL of water, what is the new concentration of the solution?
What is the final pH after adding 25 mL?