LESSON 110: Electron Cravings: Oxidation-Reduction
THINK ABOUT IT
A silvery or reddish mineral called hematite, Fe2O3, is the main source of iron metal on Earth. When this iron (III) oxide is decomposed to iron and oxygen, the oxygen atoms lose electrons. This means that the iron atoms gain electrons.
What happens to electrons during oxidation?
To answer this question, you will explore
Oxidation-Reduction
Redox Reactions
Oxidation-Reduction
EXPLORING THE TOPIC
Oxidation-Reduction

When iron oxides are decomposed to iron metal, the iron cations gain electrons and the oxygen anions lose electrons.
2Fe2O3(s) → 4Fe(s) + 3O2(g)
The oxygen anions in Fe2O3(s) have a charge of –2. In the decomposition of Fe2O3(s), each O2– anion loses two electrons to form neutral oxygen atoms. The oxygen atoms bond in pairs to form elemental oxygen, O2 (to satisfy the octet rule). In this process, the oxygen anions, O2–, are oxidized to elemental oxygen, O2.
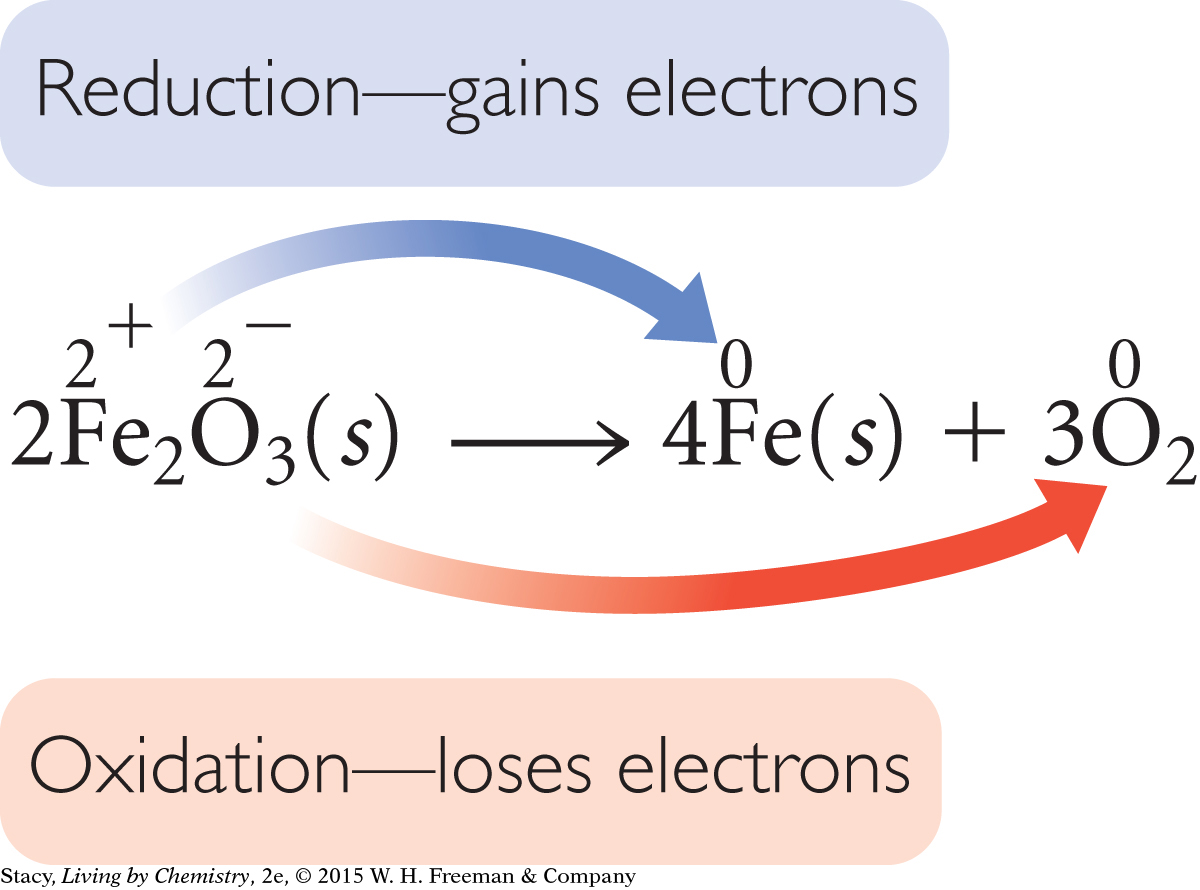
The iron cations in Fe2O3(s) have a charge of +3. The iron atoms in Fe(s) have a charge of 0. In the decomposition of Fe2O3(s), each Fe3+ cation gains three electrons to form neutral iron atoms in the elemental metal. Just as there is a term for losing electrons (oxidation), there is a term for gaining electrons: reduction. In this process, the iron atoms, Fe3+, are reduced to elemental iron, Fe.
Whenever metals are extracted from their compounds, they are reduced. While the term reduction refers to the process of gaining electrons, its origins have to do with the concept of reducing metal ores to their simplest elements. Whenever you see a metal element as the product of a reaction, you can be fairly certain that it has been reduced.
INDUSTRY CONNECTION
INDUSTRY
CONNECTION
Barium is a metal that is easily oxidized in air to form BaO. So, it is difficult to obtain this metal in its pure form. Because barium reacts so easily with oxygen, it is sometimes used to create vacuum tubes. It can remove the last of the oxygen.
Every time a substance loses electrons, another substance gains electrons and vice versa. These two processes always take place together: oxidation and reduction. Oxidation-reduction reactions are often referred to as redox reactions.
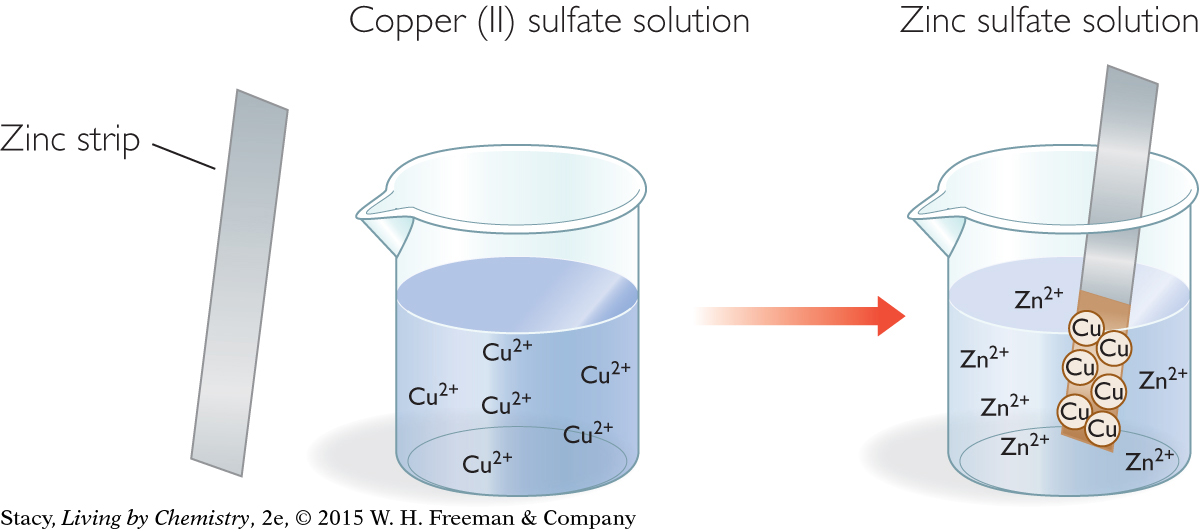
REACTION OF ZINC AND COPPER SULFATE
A strip of zinc metal is placed in a light blue solution of copper (II) sulfate, CuSO4, as shown in the illustration. Soon, a reddish coating begins to form on the zinc strip. The blue color of the solution begins to fade. What is going on?
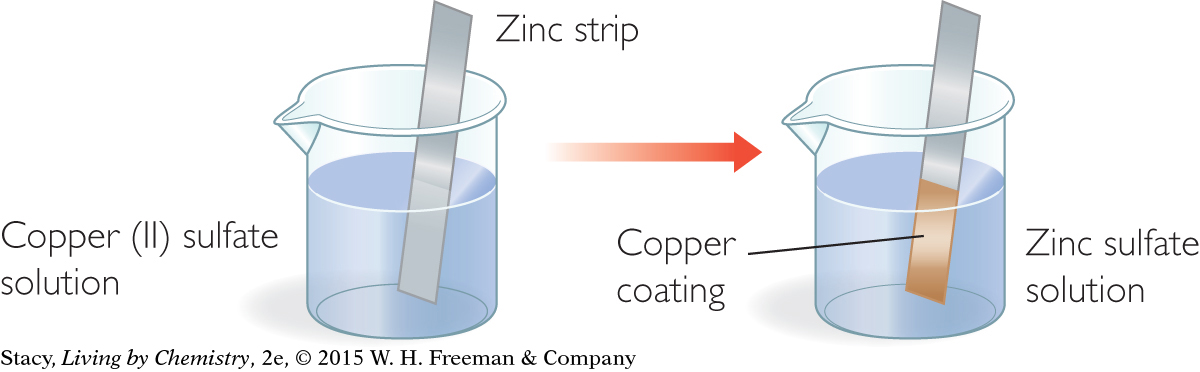
Start with the balanced chemical equation for this reaction:
Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
You know from Unit 1: Alchemy that when salts dissolve in water, the compound dissociates into ions. Often, chemical equations are written in ionic form to specify the charges of the ions in solution as shown here.
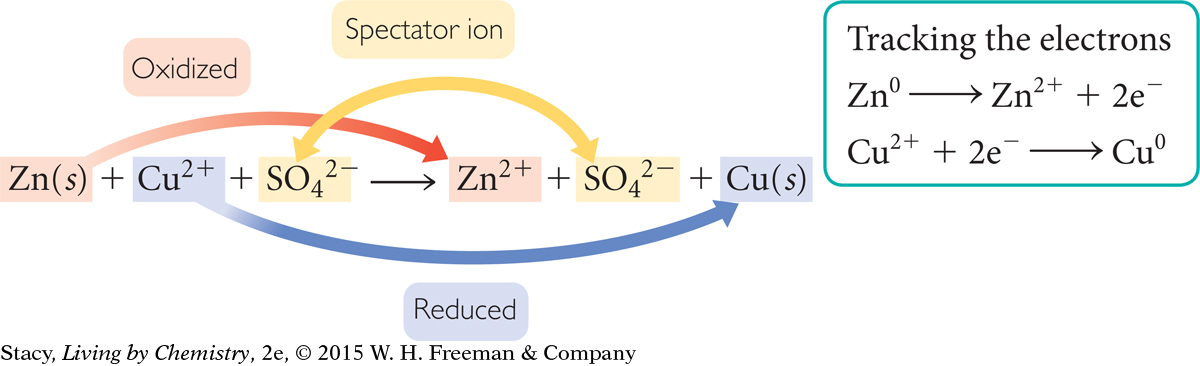
Notice that the sulfate ion is a spectator ion. The net ionic equation is therefore
Zn(s) + Cu2+ → Zn2+ + Cu(s)
The next illustration shows this reaction from a particle view. Take a moment to follow the transfer of the electrons.
Important to Know
The atom that is being reduced gains electrons. The atom that is oxidized loses electrons.
Redox Reactions
Redox Reactions
CONSUMER CONNECTION
CONSUMER
CONNECTION
When silverware comes into contact with certain foods, it is oxidized and becomes tarnished. Eggs are an example of a food item that will tarnish silver. Eggs are rich in sulfur compounds. The compound that forms as a result of this oxidation is silver sulfide, Ag2S(s).

Many chemical reactions are oxidation-reduction reactions. In fact, any reaction in which electrons are transferred from one reactant to another is a redox reaction. This includes most reactions that have an element as a reactant or product, which are often single exchange reactions.
Typically, double exchange reactions are not redox reactions. For example, in acid-base reactions, H+ trades places with another cation. In precipitation reactions, an aqueous ion from one compound combines with an aqueous ion from another. So, double exchange reactions often involve transfer of ions, while single exchange reactions involve transfer of electrons.
SINGLE EXCHANGE: REDOX REACTION
Mg(s) + 2HCI(aq) → H2(g) + MgCI2(aq)
DOUBLE EXCHANGE: ACID-BASE REACTION
NaOH(aq) + HCI(aq) → H2O(I) + NaCI(aq)
DOUBLE EXCHANGE: PRECIPITATION REACTION
AgNO3(aq) + KCI(aq) → AgCI(s) + KNO3(aq)
Oxidation-reduction reactions are not limited to metals and ionic compounds. Molecular covalent compounds also take part in oxidation-reduction reactions.
COMBUSTION: REDOX REACTION
CH4 + 2O2 → CO2 + 2H2O
In this reaction, carbon is oxidized. Electron transfer is trickier to identify in reactions with covalent compounds. But we already know that combustion is a form of oxidation. For molecules, oxidation often involves a loss of hydrogen and a gain of oxygen, and reduction often involves a gain of hydrogen and the loss of oxygen.
Example
Oxidation-Reduction
Solid magnesium, Mg, is placed in a solution of iron (III) nitrate, Fe(NO3)3. Magnesium nitrate and solid iron are formed. Show the ionic equation and determine what is oxidized and what is reduced.
Solution
Write and balance the chemical equation for this reaction:
3Mg(s) + 2Fe(NO3)3(aq) → 3Mg(NO3)2(aq) + 2Fe(s)
Translate this equation into an ionic equation. Ignore the spectator ions, in this case, the NO3– ions.
3Mg(s) + 2Fe3+ (aq) → 3Mg2+ (aq) + 2Fe(s)
Tracking the electrons
3Mg0 → 3Mg2+ + 6e–
2Fe3+ + 6e– → 2Fe0
The metal losing electrons is oxidized. The metal gaining electrons is reduced.
Each magnesium atom loses two electrons, so magnesium is oxidized. Two iron ions accept three electrons each, so iron is reduced
LESSON SUMMARY
LESSON SUMMARY
What happens to electrons during oxidation?
KEY TERMS
reduction
oxidation–reduction reaction (redox)
Whenever an atom or ion loses electrons, another atom or ion gains electrons. The loss of electrons is called oxidation, and the gain of electrons is called reduction. Oxidation and reduction always occur together. This process is known as oxidation-reduction, or redox. When metals react with oxygen to form metal oxides, the metal atoms are oxidized and the oxygen is reduced. To extract elemental metals, the reverse reaction must occur and metal ores must be reduced.
Exercises
Reading Questions
Explain what is oxidized and what is reduced when copper reacts with oxygen to form copper (II) oxide, CuO.
Explain what is oxidized and what is reduced when copper (II) oxide is decomposed to copper and oxygen.
Reason and Apply
Describe how you might extract a metal from a metal salt solution.
Determine what is oxidized and what is reduced in these reactions:
CO2(g) + H2(g) → CO(g) + H2O(g)
SF4(g) + F2(g) → SF6(g)
4Ag(s) + 2H2S(g) + O2(g) → 2Ag2S(s) + 2H2O(g)
C6H12O6(aq) + 6O2(g) → 6CO2(g) + 6H2O(l)
Write the net ionic equations for these reactions:
Zn(s) + CuCl2(aq) → Cu(s) + ZnCl2(aq)
Ba(NO3)2(aq) + CuSO4(aq) → BaSO4(s) + Cu(NO3)2(aq)
Determine which of the reactions in Exercise 5 is not a redox reaction. Explain your reasoning.
Which of these reactions from Unit 4: Toxins can be classified as redox reactions? For each redox reaction, show what is oxidized, what is reduced, and the total number of electrons transferred.
Tl2O(s) + 2HCl(l) → 2TlCl(aq) + H2O(l)
HgS(s) + 2HCl(aq) → HgCl2(s) + H2S(aq)
PbCO3(s) + 2HCl(aq) → PbCl2(aq) + H2CO3(aq)
Na2C2O4(aq) + CaCl2(aq) → CaC2O4(s) + 2NaCl(aq)
Pb(s) + 2HCl(aq) → PbCl2(aq) + H2(g)
2As(s) + 6HCl(aq) → 2AsCl3(aq) + 3H2(g)