LESSON 111: The Active Life: Activity of Metals
THINK ABOUT IT
Imagine that you set up three experiments. In one, a strip of copper is placed in a solution of silver nitrate. In the second, a strip of zinc is placed in a solution of copper (II) nitrate. In the third, a strip of silver is placed in a solution of zinc nitrate. Only two of these setups will result in reactions. How can you predict which reactions will occur?
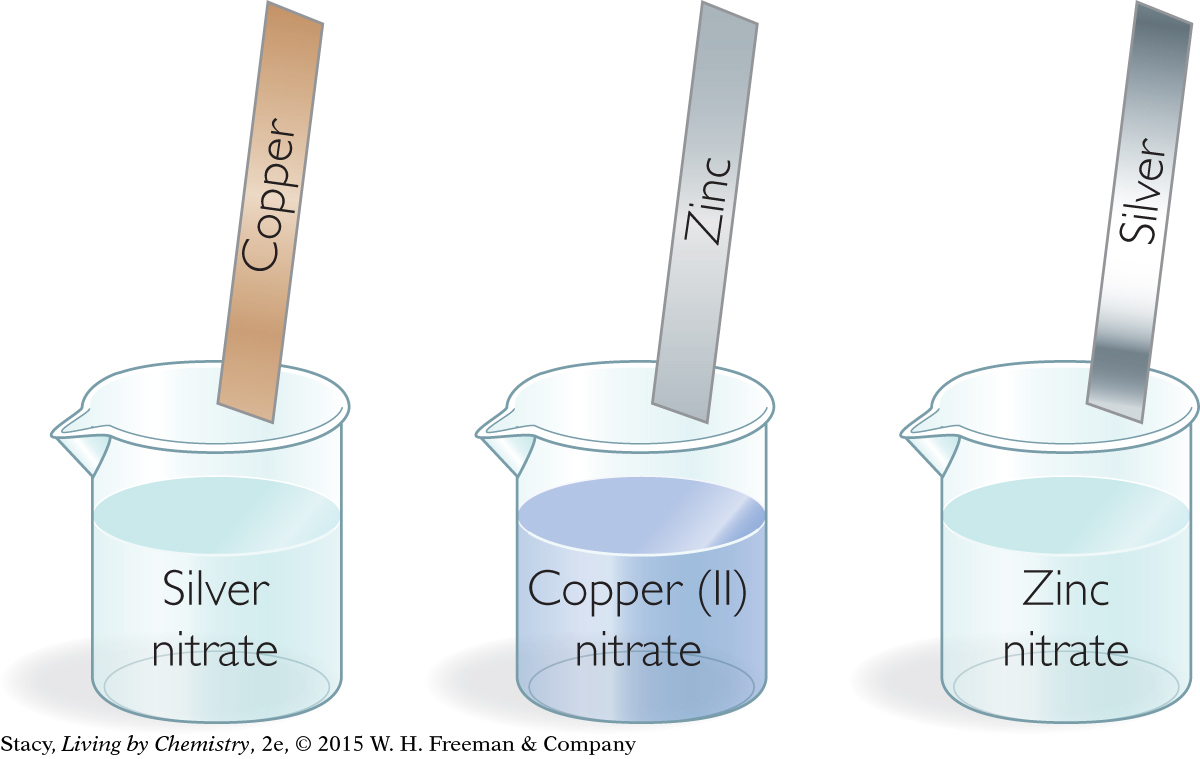
Which metal atoms are most easily oxidized?
To answer this question, you will explore
Comparing Metals
Activity Series
Comparing Metals
EXPLORING THE TOPIC
Comparing Metals
INDUSTRY CONNECTION
INDUSTRY
CONNECTION
Redox reactions have been used for decades to coat metals with chromium. Chrome plating improves appearance and durability. Chromium sulfate, or chromium chloride, is often used in industrial plating solutions.

When metal atoms are combined with metal ions in solution, you may or may not end up with a reaction. Some combinations of metals and metal salts result in redox reactions and others don’t. It all depends on which combinations of metals are used.
Consider the three solutions shown on the next page. Each solution contains metal cations and a strip of elemental metal. If the metal atoms on the strip transfer electrons to the metal cations in solution, a reaction will occur.
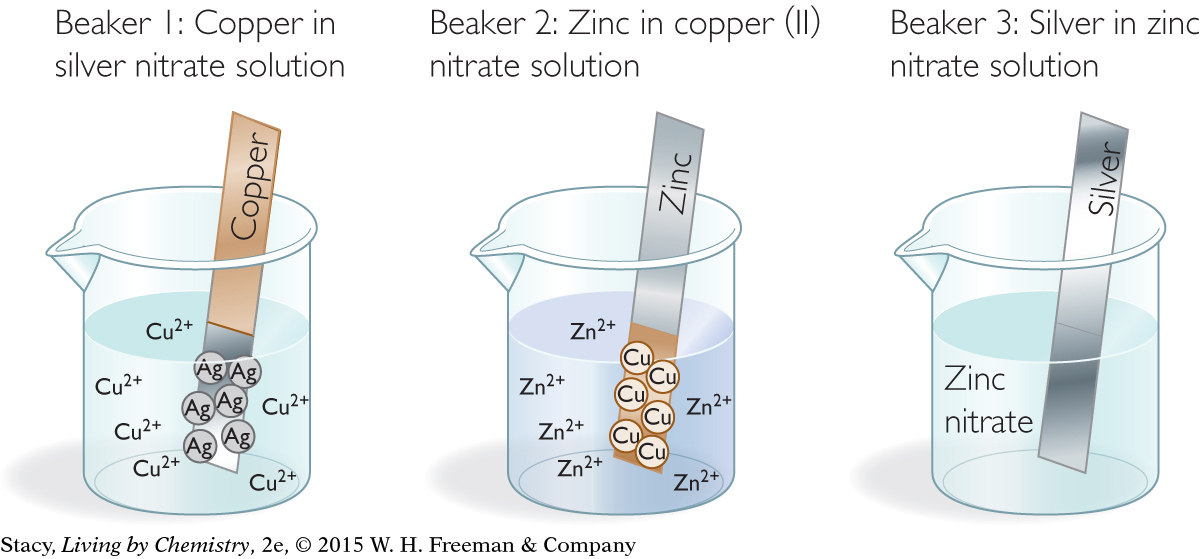
Beaker 1: Cu(s) + 2AgNO3(aq) → Cu(NO3)2(aq) + 2Ag(s)
CONSUMER CONNECTION
CONSUMER
CONNECTION
Lithium is the most active metal on the periodic table and would potentially be listed above potassium on the activity series table. Your cell phone may contain a lithium battery. The chapter-opening photo shows a lithium ion battery in an electric car. A redox reaction in the battery supplies electrical energy to the car’s motor.

Cu(s) + 2Ag+(aq) → Cu2+(aq) + 2Ag(s)
Beaker 2: Zn(s) + Cu(NO3)2(aq) → Zn(NO3)2(aq) + Cu(s)
Zn(s) + Cu2+(aq) → Zn2+(aq) + Cu(s)
Beaker 3: Ag(s) + Zn(NO3)2(aq) → no reaction
In the first beaker, the copper atoms transfer electrons to the silver ions. Copper atoms are oxidized and silver cations are reduced. As a result, silver metal can be seen as a coating on the copper strip. In the second beaker, zinc atoms transfer electrons to the copper ions in solution. Zinc atoms are oxidized and copper is reduced. Copper metal coats the zinc strip. However, nothing happens in the third beaker.
In the third beaker, there is no reaction. Silver does not give up its electrons in the presence of zinc ions. Chemists say that silver is not as active as zinc.
Activity Series
Activity Series
By combining different metals and metal ions, you can determine experimentally which metals are more active than other metals. The result is called an activity series. Working with the data we have so far, zinc, copper, and silver can be placed in order of their activity.

Where would magnesium fit on this list? In the lab, you combined magnesium with zinc nitrate. The magnesium was oxidized and formed Mg2+ ions.
Mg(s) + Zn(NO3)2(aq) → Mg(NO3)2(aq) + Zn(s)

So, magnesium belongs above zinc on the list.

You may have noticed that all the reactions in this section have been single exchange reactions in which the more easily oxidized metal displaces the other metal. In this way, the more active metal forms cations while the less active metal is reduced to a solid.
A series of experiments can reveal where other metals belong on the list. The more active atoms at the top of the list will always displace the less active ones below.
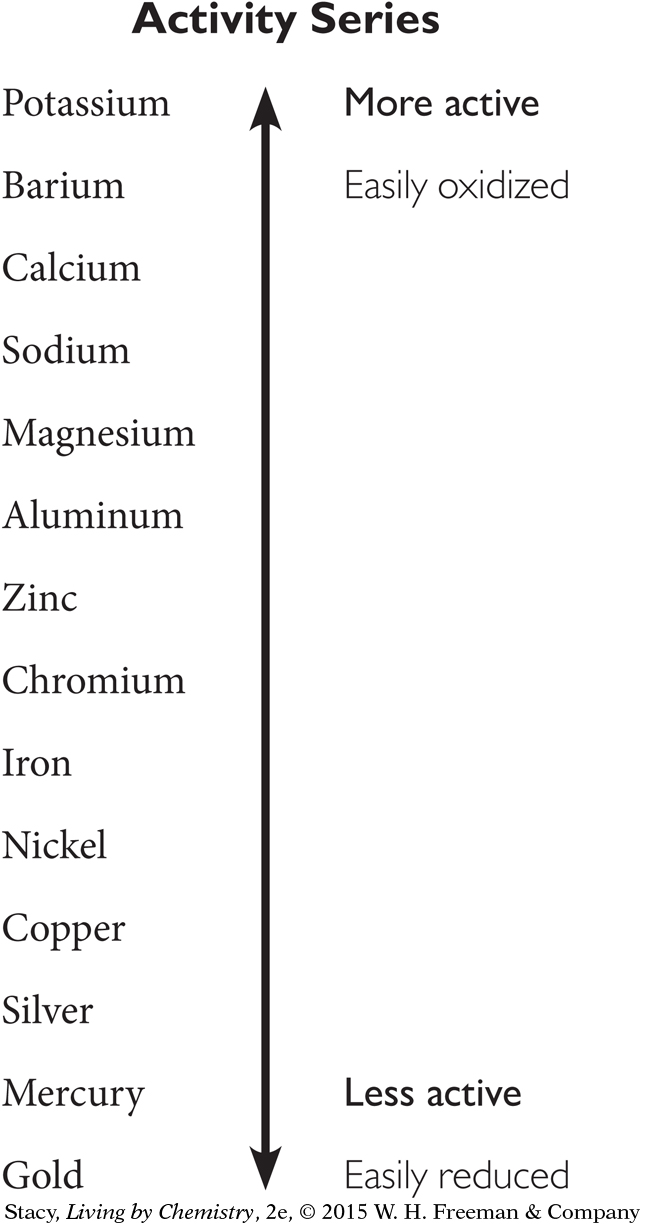
On this list, gold is at the bottom. Gold atoms are very difficult to oxidize, and gold cations are very easy to reduce. This is one reason why gold is rarely found in compounds combined with other atoms.
A metal high in the activity series
reacts vigorously with compounds
readily gives up electrons in reactions to form positive ions
is corroded easily
A metal low in the activity series
does not react vigorously with chemicals
does not readily give up electrons in reactions to form positive ions
is not corroded easily
Example
Activity of Lead
Lead, Pb, will give up electrons to copper cations, Cu2+, but not to zinc cations, Zn2+.
Write the net ionic equation for the reaction between Pb and Cu2+.
Do you expect that Zn will transfer electrons to lead cations, Pb2+? Explain your reasoning.
Solution
Pb(s) + Cu2+(aq) → Pb2+(aq) + Cu(s)
Pb does not give up electrons to Zn2+. This means that the reverse reaction will happen. Zn will give up electrons to Pb2+. Zn is more active than Pb, so it displaces Pb in the ionic compound.
Zn(s) + Pb2+(aq) → Zn2+(aq) + Pb(s)
All of the metals on the periodic table can be placed in an activity series table. Chemists are interested in the activity of metals, because this allows them to control the outcome of different reactions. Metal compounds can be combined in different ways to form other compounds. Also, the most easily oxidized metals turn out to be a good source of electrons. You’ll learn more about this in Lesson 112: Current Events.
LESSON SUMMARY
LESSON SUMMARY
Which metal atoms are most easily oxidized?
KEY TERM
activity series
Metals that are easily oxidized give up their electrons easily. They are considered more active than other metals. Comparing metals experimentally allows you to rank the metals in terms of their ease of oxidation. The activity of a metal is demonstrated when it is combined with another metal cation. The more active metal will lose electrons, displacing the other metal in the compound. The displaced metal is reduced. The ranking of metals in order of their activity is referred to as an activity series.
Exercises
Reading Questions
Explain how you would figure out where tin should be listed in the activity series.
Why are metals that are more active also considered less stable?
Reason and Apply
If zinc nitrate, Zn(NO3)2, is paired with solid iron, what would you expect to observe? Explain your reasoning.
If iron (III) nitrate, Fe(NO3)3, were combined with solid zinc, what would you expect to observe? Explain your reasoning.
Consider any reactions that occur in Exercises 3 and 4.
Write chemical equations.
Write the net ionic equation.
Identify the more active metal.
Which metal is oxidized? Which metal is reduced?
Platinum is more active than gold but less active than silver. Where does it belong in the activity series?
If cobalt ions are replaced by iron to form iron ions and solid cobalt, is cobalt above or below iron in the activity series? Explain.
Write net ionic equations for two reactions between magnesium and less active metals.
Name three combinations of ionic compounds and metals that will not react.