LESSON 118: How Backward
THINK ABOUT IT

Imagine that you open a bottle of perfume. You detect a sweet smell that remains quite intense as long as the bottle is open. This indicates that molecules are attaching to receptors in your nose and causing a sensation by stimulating nerves that send signals to your brain. Once you close the bottle, the sweet smell grows fainter over time. Why don’t you continue to detect the sweet smell?
What is a reversible process?
To answer this question, you will explore
Reversible Processes: The Double Arrow
Examples of Reversible Processes
Degree of Sweetness
Reversible Processes: The Double Arrow
EXPLORING THE TOPIC
Reversible Processes: The Double Arrow
Based on what you have learned so far in this course, it might seem that chemical processes proceed only in the direction in which they are written as a chemical equation. For example, here is the chemical equation that describes gasoline burning.
2C8H18(l) + 25O2(g) → 16CO2(g) + 18H2O(g)
When gasoline burns, the reaction goes in the forward direction, from reactants to products. This reaction is said to go to completion, which means that all reactants are completely converted to products. Although it is possible for gasoline to be created through chemical reactions, it takes many chemical steps and requires a great deal of energy. So this reaction, as it is written, is not reversible. Once you burn the gasoline, there is no going back.
In contrast, there are many reversible processes that proceed in both the forward and reverse directions. The term process can describe either a physical process or a chemical process (chemical reaction). In Lesson 70, you learned that chemical equations are used to represent both chemical and physical processes.
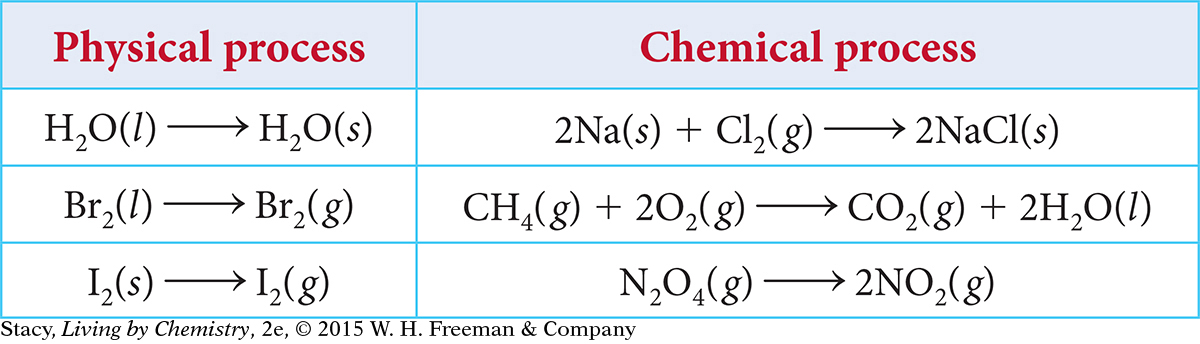
As written, the processes in the table appear to move in one direction only. However, some of these processes are reversible.
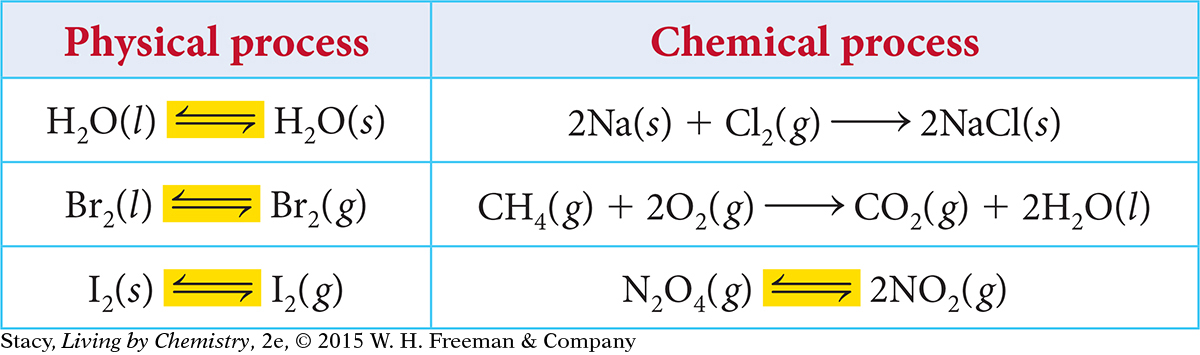
Notice that for a reversible process, a double arrow is used in the equation. The double arrow indicates that both a forward and a reverse process can occur. A reversible process can be written in either the forward or reverse direction. All phase changes are reversible, while many chemical processes are not.
Big Idea
Big Idea
All physical processes and some chemical processes are reversible.
Examples of Reversible Processes
Examples of Reversible Processes

As is evident from the second table above, phase changes are reversible. Other common reversible processes involve processes in aqueous solution, such as dissolving and precipitation and acid-base reactions, and processes in the human body involving binding to a receptor. Reversible processes can occur in both the forward and reverse directions. Depending on the conditions, it is possible to cause these processes to proceed exclusively in one direction, or to create a mixture of starting substances and products. This is explained further with the examples below.
PHASE CHANGES
Consider water boiling in a pot on the stove. The liquid water evaporates and spreads out in the air. If the gaseous water is cooled, the reverse process occurs and water droplets form. Intermolecular forces, including hydrogen bonds and dipole-dipole attractions, account for the attraction between water molecules. Heating easily breaks these forces of attraction, and they re-form by cooling. One way to represent this reversible process of evaporating and condensing is with a double arrow as shown in the equation below.
H2O(l) ⇋ H2O(g)
DISSOLVING AND PRECIPITATION

Suppose you dissolve a small amount of salt in water to create an aqueous salt solution. If you evaporate the water, the reverse process occurs and solid salt precipitates. The dominant force that accounts for the formation of the solid is cation-anion attractions. Upon dissolving, the ions are attracted to the water molecules by ion-dipole attractions. The difference in the strength of these forces is small enough such that the reaction is easy to reverse. One way to represent this reversible process of dissolving and precipitating is with a double arrow as shown in the chemical equation below.
NaCl(s) ⇋ Na+ (aq) + Cl−(aq)
ACID-BASE REACTIONS
When an acid is dissolved in water, the acid molecules dissociate into H+ and an anion. This process can also go in the reverse direction, in which the ions are attracted to one another to form the acid molecule, depending on the pH of the solution.

In the previous lesson, you examined a solution of an acid-base indicator, HIn. Adding base to an aqueous solution causes the HIn molecules to dissociate into H+ and In− ions. Adding acid causes the reverse process in which the ions combine to form HIn molecules.
A solution of the indicator bromothymol blue is yellow in acidic solutions due to the presence of mostly HIn molecules, blue in basic solutions due to the presence of mostly In− anions, and green at pH = 7 because both HIn molecules and In− anions are present in roughly equal amounts. A double arrow, as shown in the chemical equation below, is one way to represent the reversible process of acid molecules dissociating into ions and ions attracting one another to re-form molecules.
HIn(aq) ⇋ H+(aq) + In−(aq)
BINDING TO A RECEPTOR IN THE BODY
Processes involving binding to receptor sites in the human body are reversible. Prime examples include taste and smell, in which molecules attach to and reversibly release from receptors on the tongue and in the nose. The receptors are made of large protein molecules with a specific shape and sections that are polar, charged, or nonpolar.
Small molecules are attracted selectively to the protein by intermolecular attractions and how well the molecule fits. When molecules attach to the receptor, this initiates a signal sent along the nervous system to the brain. The brain receives the signal and it is perceived as a certain taste or smell. The signal is no longer detected when the molecules leave the receptor.
The general process for reversible binding between a molecule and a receptor is shown below. The blue shape represents a small molecule that is soluble in blood, saliva, or fluids in the cell. The red shape is a piece of a large protein molecule that forms the receptor. In the forward process, the molecule attaches to the receptor. In the reverse process, the molecule separates from the receptor.
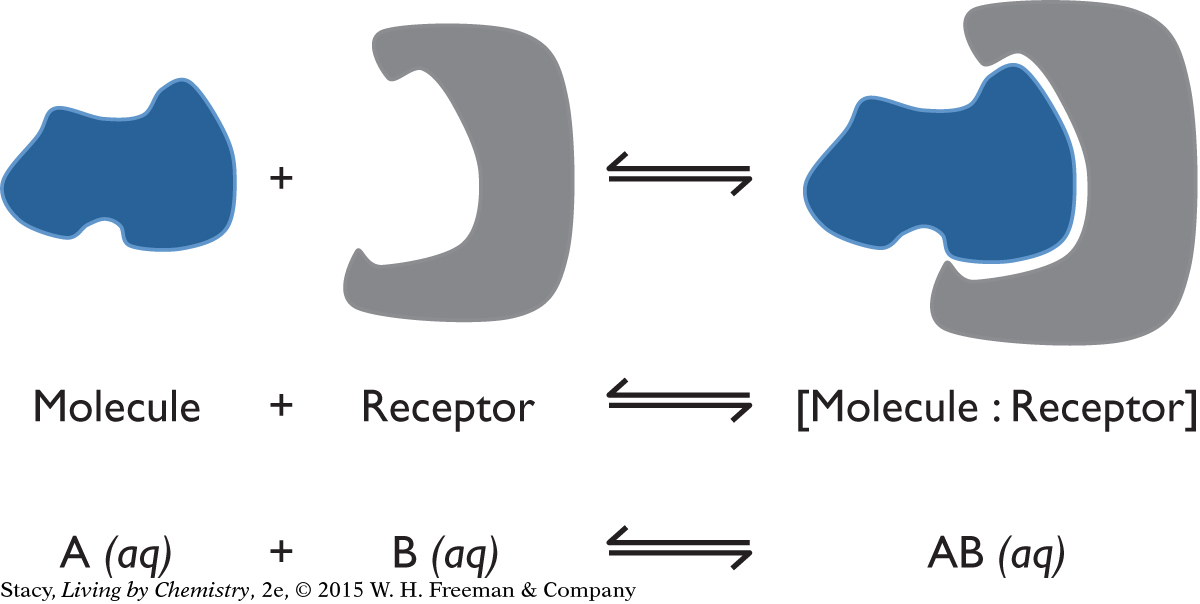
There are many reversible receptor processes in the body. The smell of menthol is detected in the nose when menthol molecules are attracted to smell receptor sites. A protein in the blood called hemoglobin reversibly binds oxygen, O2. The pain reliever ibuprofen binds reversibly to an enzyme to block it from producing molecules that cause the pain signal. The pain signal returns as the ibuprofen molecules leave the receptor. In the chemical equations below, the symbol “:” represents an attraction between the molecule and receptor.
menthol + receptor ⇋ [menthol : receptor]
O2 + hemoglobin ⇋ [O2 : hemoglobin]
ibuprofen + receptor ⇋ [ibuprofen : receptor]
Some of the reversible processes described above can be represented as two components, A and B, forming one component, AB, and then breaking apart as A and B. Here is a general form of a chemical equation describing this type of process:
Important to Know
If a process is reversible, the equation describing it can be written in either the forward or reverse direction.
A + B ⇋ AB
Because this describes a reversible process, the equation can also be written in the other direction:
AB ⇋ A+ B
This model can be used to describe salt dissolving and precipitating, acid-base reactions, and molecules binding to and releasing from receptors. For example, for salt dissolving, AB represents the salt, and A and B are the ions that it separates into when dissolved. For sugar molecules binding to a taste receptor, A represents a sugar molecule and B represents the receptor.
Degree of Sweetness
Degree of Sweetness
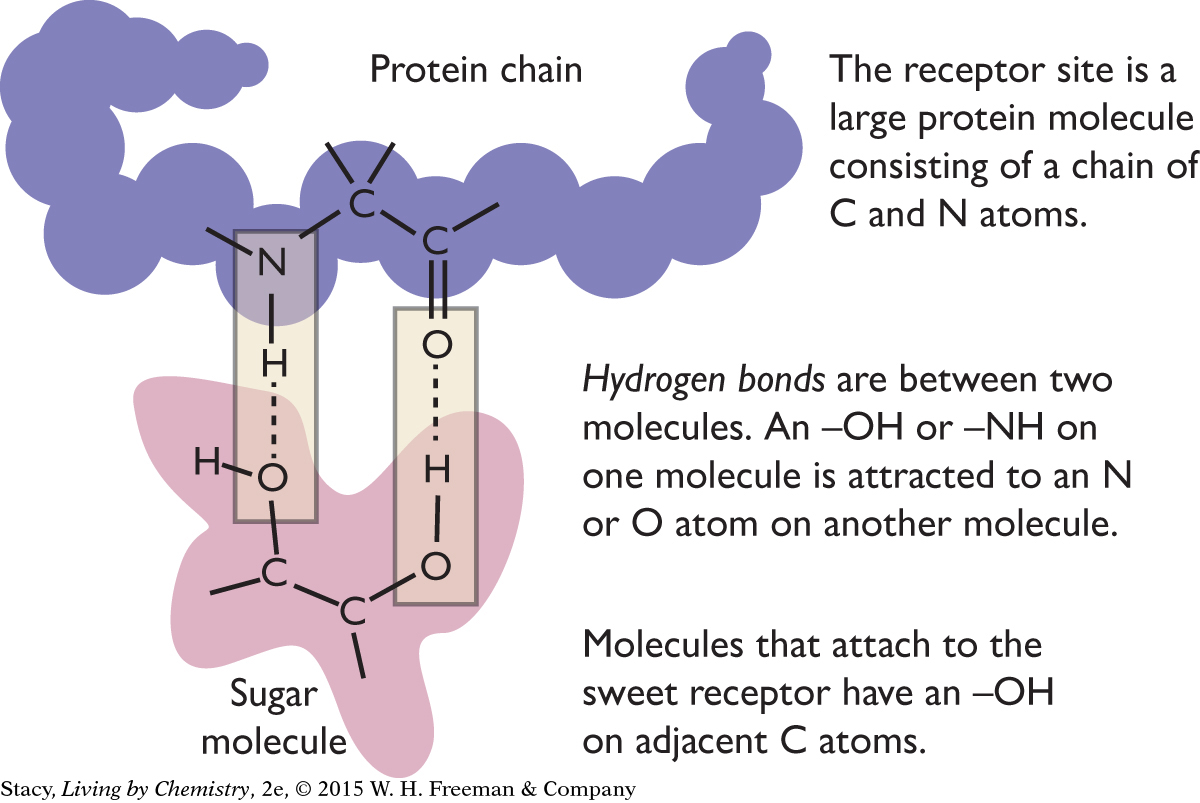
According to the receptor site theory, the attraction of sugar molecules to sweet receptors in the taste buds on the tongue involves the formation of two hydrogen bonds on two adjacent carbon atoms in the sugar molecule. Recall from Unit 2: Smells that a hydrogen bond forms when an O–H or N–H on one molecule is attracted to an O or N atom on another molecule.

Joy Brown/Shutterstock
|
|
NATURE CONNECTION
NATURE
CONNECTION
Pointed stalactites and stalagmites form when limestone alternately dissolves in and precipitates out of the dripping water in a cave. These calcite structures build slowly, at the rate of about one quarter inch to one inch per century.

The table below shows the relative sweetness for four substances. The molecules in these substances all attach to the same type of sweet receptors. Why do they vary in relative sweetness?
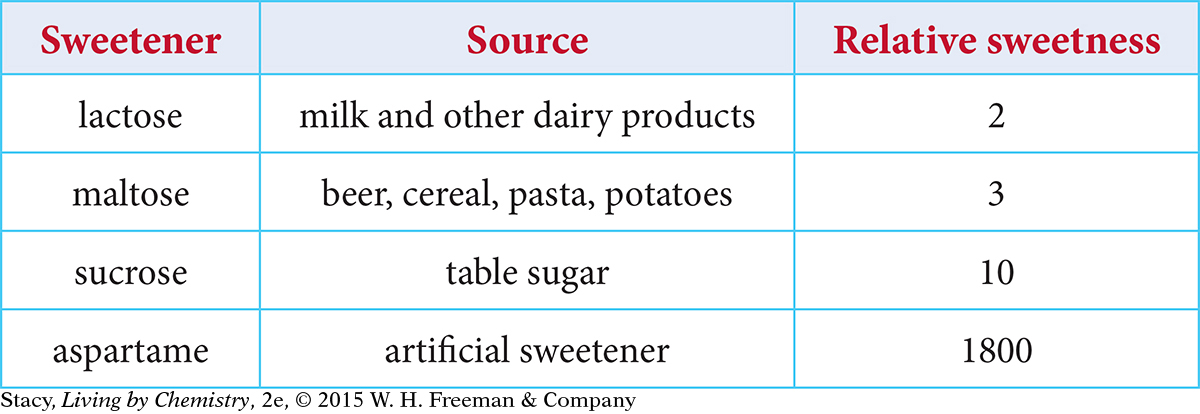
One explanation for the data is that some molecules stay in the sweet receptor longer, thereby creating a stronger sensation. This can be explained by differences in the strength of the intermolecular attractions between the molecule and the receptor, as well as a better fit. Sucrose (table sugar) is attracted more strongly to a sweet receptor than maltose (the sugar in malt) and more strongly still than lactose (the sugar in milk). The result is that, for the same number of molecules and the same number of sweet receptors, more sucrose molecules are detected because more are attached to the sweet receptors. For lactose, more molecules remain in the saliva.
Artificial sweeteners are about 180 times sweeter than table sugar (sucrose) per mole. This is why packets of artificial sweeteners are much smaller than packets of table sugar.
Big Idea
Big Idea
Some reversible processes favor the forward direction, while others favor the reverse direction.
LESSON SUMMARY
LESSON SUMMARY
What is a reversible process?
KEY TERM
reversible process
A reversible process is one that can proceed in both the forward and the reverse directions. Many physical and chemical processes are reversible given the appropriate conditions. In a chemical equation, a double arrow is used to symbolize reversible processes. Examples of reversible processes include phase changes, dissolving and precipitation, acid-base reactions, and binding to protein receptors. In each case, it is possible to find conditions to cause these processes to proceed almost exclusively in one direction or the reverse, or to create a mixture of starting substances and products.
Exercises
Reading Questions
What evidence is there that some processes are reversible?
What does a double arrow in a chemical equation tell you?
Reason and Apply
Provide examples to support the claim that phase changes are reversible.
Provide examples to support the claim that processes involving the breaking and forming of intermolecular attractions are reversible.
Provide examples to support the claim that processes involving breaking and making of several covalent bonds are sometimes not reversible.
Imagine that you take an ice cube out of the freezer and place it on a counter at room temperature.
Write a balanced equation for the process that occurs.
Describe what you would do to reverse the process.
Imagine that you dissolve sucrose, C12H22O11(s), in water.
Write a balanced equation for this reversible process.
In which direction does the process proceed if you place a tiny amount of sugar in a large pot filled with water?
In which direction does the process proceed if you allow the water to evaporate from a sugar solution?
Methane, CH4(g), burns in oxygen, O2(g), to produce carbon dioxide, CO2(g), and water, H2O(l).
Write a balanced equation for this process.
This process is described as irreversible. What does this mean?
Imagine that you breathe in oxygen and it binds to hemoglobin in your blood to form [O2 : hemoglobin].
Write a balanced equation for the process.
The [O2 : hemoglobin] delivers oxygen to your cells. Describe this process.
Provide evidence that this process is reversible.