LESSON 21: Salty Eights: Formulas for Ionic Compounds
THINK ABOUT IT
HISTORY CONNECTION
HISTORY
CONNECTION
Salt has been a valuable trade item since ancient times. For thousands of years—before refrigeration—humans depended largely on salt as a preservative for foods, especially meat and fish. The wealth of a city, country, or individual could often be linked to the amount of salt possessed or stored. People who are “worth their salt” are considered worthwhile and valuable. Salzburg, Austria, shown here, is named for its many salt mines, which contributed to its great wealth.

There are at least 50 common metal elements. These combine with over 15 nonmetal elements to make a wide variety of ionic compounds. The task of predicting compounds might seem complex given the number of possible ways to combine a metal and a nonmetal. However, if you know how many valence electrons each atom has, you can reliably predict which ionic compounds can be made.
How can you predict chemical formulas and name ionic compounds?
To answer this question, you will explore
Predicting Chemical Formulas
Naming Ionic Compounds
Predicting Chemical Formulas
EXPLORING THE TOPIC
Predicting Chemical Formulas
An ionic compound that is made up of only one kind of metal atom and one kind of nonmetal atom is known as a salt. Table salt, NaCl, is the most familiar example. To predict formulas for salt compounds, follow these guidelines:
Make sure you are combining two different elements—a metal and a nonmetal.
No matter how many atoms you use, the total number of valence electrons must add up to 8, 16, 24, or another multiple of 8.
Make sure that the charges on the metal cations and nonmetal anions in your ionic compound add up to zero (rule of zero charge).
This process applies only to the main group elements. Ionic compounds using transition elements are covered in Lesson 23: Alchemy of Paint.
Example 1
Practice Writing Chemical Formulas
Each of these cards represents an atom.
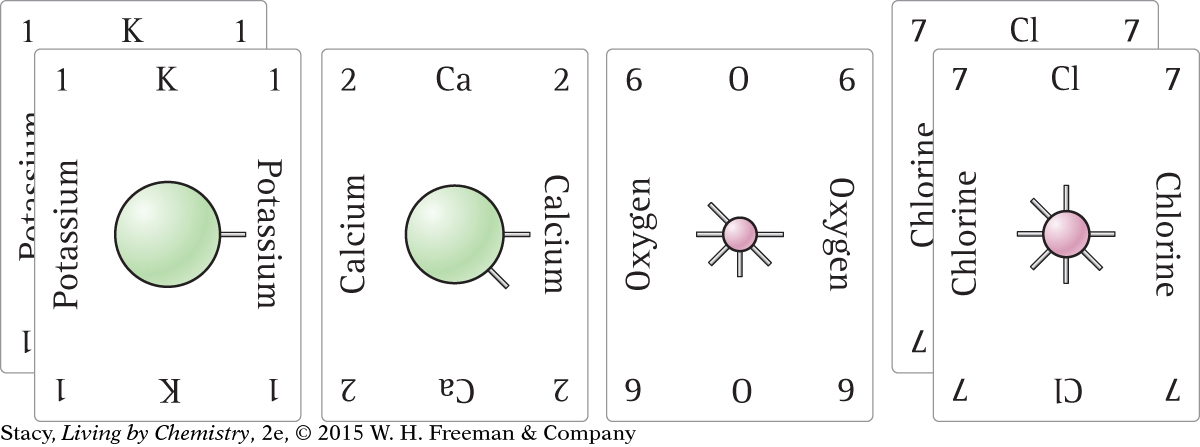
Which atoms are metals and which are nonmetals? How can you tell?
What ionic compounds can you make from two of these cards?
What ionic compounds could you make from three cards but only two different elements?
Solution
The atoms from the left side of the periodic table, with fewer valence electrons, are the metals. So, potassium, K, and calcium, Ca, are metal atoms. The atoms from the right side of the periodic table, which have closer to eight valence electrons, are the nonmetals. These include oxygen, O, and chlorine, Cl.
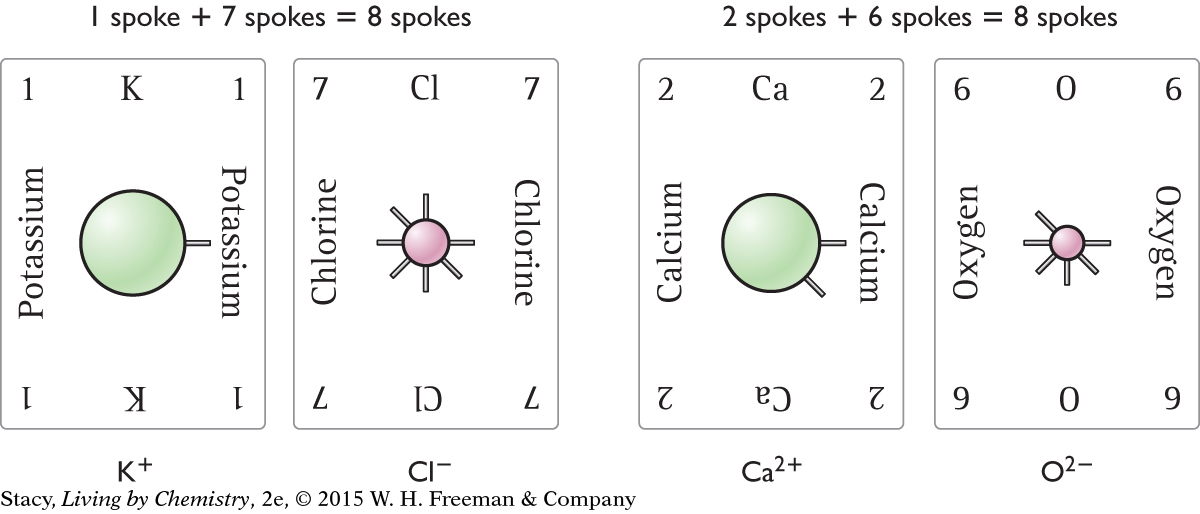
Look at the number of spokes on each card. The number of spokes represents the number of valence electrons. To create ionic compounds, combine a metal with a nonmetal. The number of spokes must add up to 8. Two ionic compounds are possible with these cards. These are shown here.
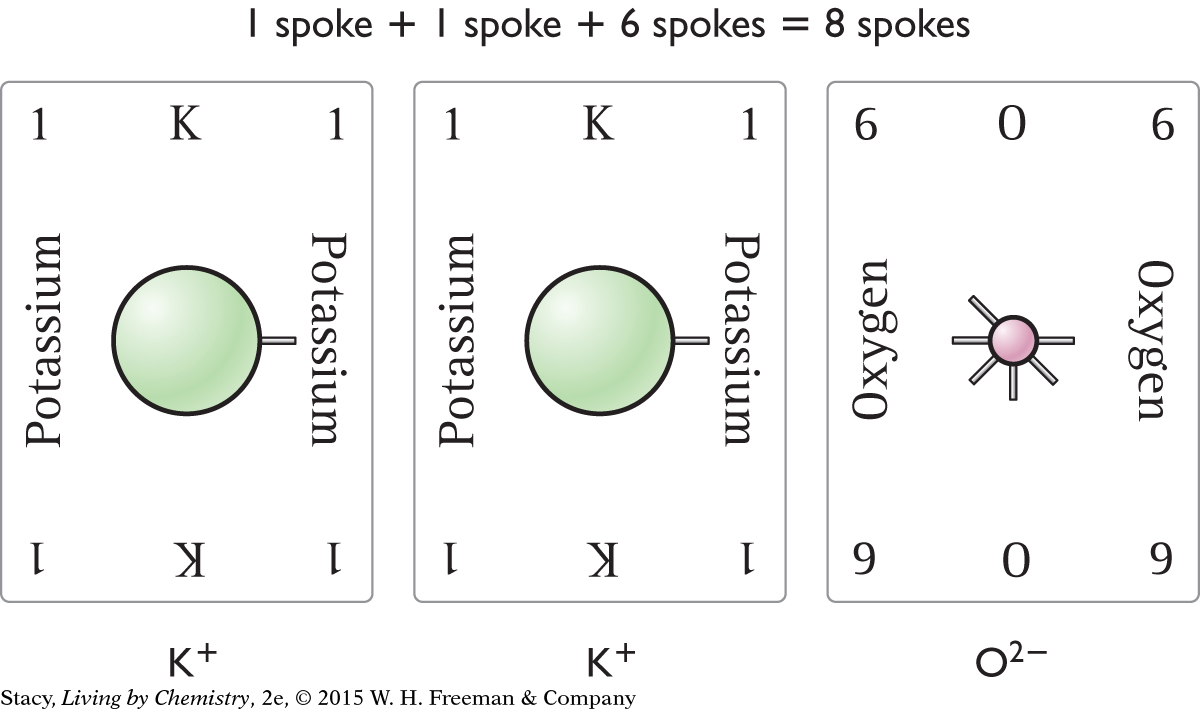
If two atoms with one valence electron combine with one atom with six valence electrons, the result is eight valence electrons. One possible compound is shown.
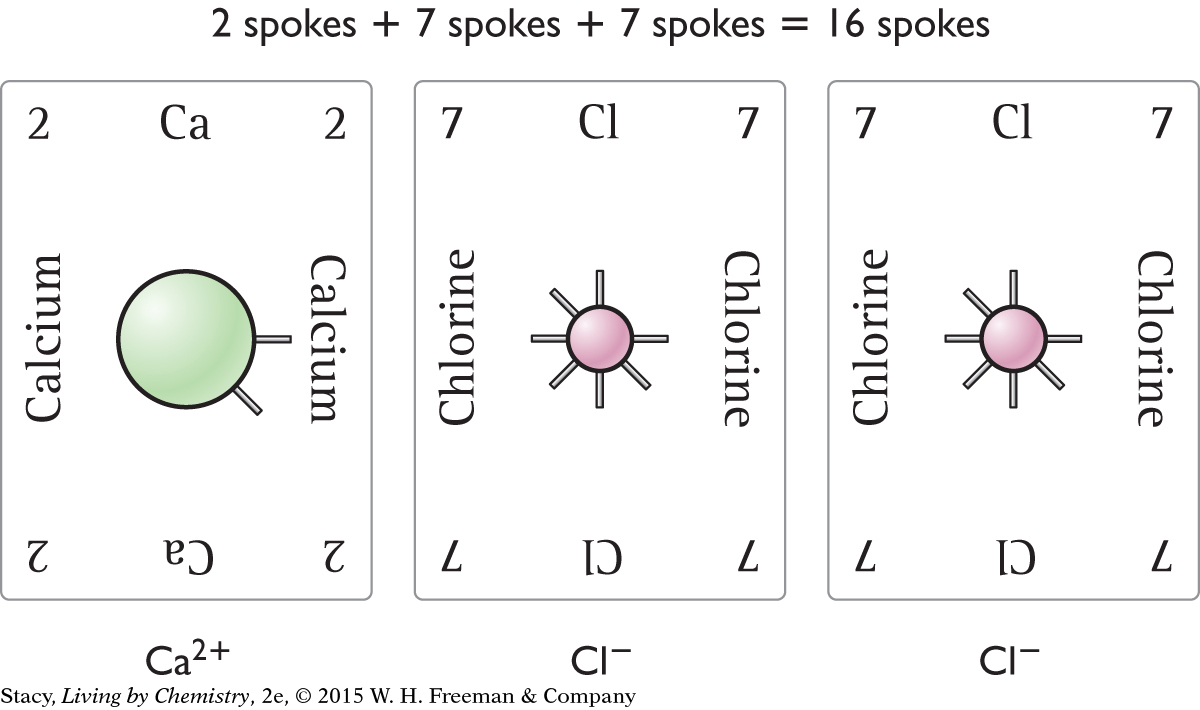
 Page 106
Page 106You can also make CaCl2. Notice that the valence electrons add up to 16, which is a multiple of 8. The charges add up to zero.
Naming Ionic Compounds
Naming Ionic Compounds
INDUSTRY CONNECTION
INDUSTRY
CONNECTION
Potassium bromide is currently in use as an anti-seizure medication for dogs and cats with epilepsy.

In the names of ionic compounds, the name of the metal atom comes first, followed by the name of the nonmetal atom. However, the name of the nonmetal atom is altered slightly by replacing the last part of the word with the letters “-ide.” Here is a list of ionic compounds with their names. Take a moment to examine them.
| Compound | Name | Compound | Name |
| NaCl | sodium chloride | Al2O3 | aluminum oxide |
| MgF2 | magnesium fluoride | GaP | gallium phosphide |
| Li2S | lithium sulphide |
So in ionic compounds, sulfur becomes sulfide, nitrogen becomes nitride, and bromine becomes bromide.
Notice that the chemical name for ionic compounds does not have anything to do with the subscript numbers in the chemical formula. For example, MgCl2 is simply magnesium chloride, not magnesium dichloride.
Example 2
Naming Compounds
Write the chemical formula and name for the compound created from each pair of elements.
potassium and bromine
oxygen and calcium
oxygen and potassium
sodium and chlorine
sodium and oxygen
Solution
To write the formula for the compound, make sure the atoms are in the correct ratio so that the compound follows the rule of zero charge. To name the compound, write the metal atom first, followed by the nonmetal atom. Change the ending of the nonmetal atom’s name to “-ide.”
KBr, potassium bromide
CaO, calcium oxide
K2O, potassium oxide
NaCl, sodium chloride
Na2O, sodium oxide
LESSON SUMMARY
LESSON SUMMARY
How can you predict chemical formulas and name ionic compounds?
There are several important guidelines to follow in creating ionic compounds. Metal atoms are combined with nonmetal atoms. Next, the total number of valence electrons adds up to eight or a multiple of eight. Finally, the charges on the metal cations and nonmetal anions in ionic compounds add up to zero. When naming ionic compounds made from two different types of elements, the name of the metal atom comes first, followed by the name of the nonmetal atom. In addition, the ending of the name of the nonmetal atom is changed to “-ide.”
Exercises
Reading Questions
Explain how to use the periodic table to determine the charges on ions.
Explain how to use the periodic table to determine the correct formulas for ionic compounds.
Reason and Apply
Which chemical formulas are consistent with the guidelines for creating ionic compounds? Explain your thinking.
LiCl
LiCl2
MgCl
MgCl2
AlCl3
Give examples of six ionic compounds with a metal-to-nonmetal ratio of 1:1. Specify the total number of valence electrons for each compound. Name each compound.
Give examples of three ionic compounds with a metal-to-nonmetal ratio of 2:1. Specify the total number of valence electrons for each compound. Name each compound.
Give examples of three ionic compounds with a metal-to-nonmetal ratio of 1:2. Specify the total number of valence electrons for each compound. Name each compound.
Page 108Predict the formulas for the ionic compounds that are formed when these metal and nonmetal elements are combined. Name each compound.
Al and Br
Al and S
Al and As
Na and S
Ca and S
Ga and S
For each compound, write the cation and anion with the appropriate charge. Then write the chemical formula for each compound.
Example: sodium fluoride, Na+, F–, NaF
magnesium oxide
rubidium bromide
strontium iodide
beryllium fluoride
aluminum chloride
lead sulfide