ATP-Powered Ion Pumps Generate and Maintain Ionic Gradients Across Cellular Membranes
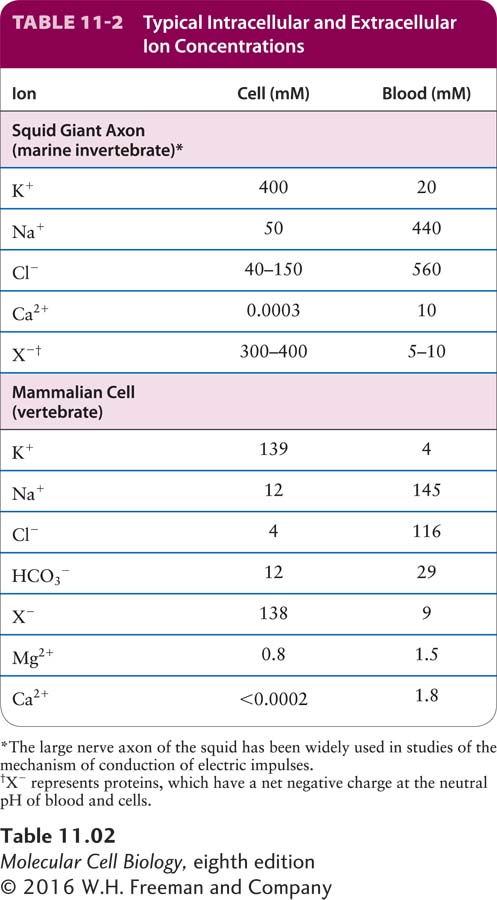
The specific ionic composition of the cytosol usually differs greatly from that of the surrounding extracellular fluid. In virtually all cells—
Some Ca2+ in the cytosol is bound to the negatively charged groups in ATP and in proteins and other molecules, but it is the concentration of unbound (or “free”) Ca2+ that is critical to its functions in signaling pathways and muscle contraction. The concentration of free Ca2+ in the cytosol is generally less than 0.2 micromolar (2 × 10−7 M), a thousand or more times lower than that in the blood. Plant cells and many microorganisms maintain similarly high cytosolic concentrations of K+ and low concentrations of Ca2+ and Na+, even if the cells are cultured in very dilute salt solutions.
The ion pumps discussed in this section are largely responsible for establishing and maintaining the usual ionic gradients across the plasma and intracellular membranes. In carrying out this task, cells expend considerable energy. For example, up to 25 percent of the ATP produced by nerve and kidney cells is used for ion transport, and human erythrocytes consume up to 50 percent of their available ATP for this purpose; in both cases, most of this ATP is used to power the Na+/K+ pump (see Figure 11-3). The resultant Na+ and K+ gradients in neurons are essential for their ability to conduct electrical signals rapidly and efficiently, as we detail in Chapter 22. Certain enzymes required for protein synthesis in all cells require a high K+ concentration and are inhibited by high concentrations of Na+; these enzymes would cease to function without the operation of the Na+/K+ pump. In cells treated with poisons that inhibit the production of ATP (e.g., 2,4-
Page 486