Only Gases and Small Uncharged Molecules Cross Membranes by Simple Diffusion
With its dense hydrophobic core, a phospholipid bilayer is largely impermeable to water-
The diffusion rate of any substance across a pure phospholipid bilayer is proportional to its concentration gradient across the bilayer and to its hydrophobicity and size; the movement of charged molecules is also affected by any electric potential across the membrane. When a pure phospholipid bilayer separates two aqueous spaces, or “compartments,” membrane permeability can be easily determined by adding a small amount of labeled material to one compartment and measuring its rate of appearance in the other compartment. The label can be radioactive or nonradioactive—
Page 475
The hydrophobicity of a substance is determined by measuring its partition coefficient K, the equilibrium constant for its partition between oil and water. The higher a substance’s partition coefficient (the greater the fraction found in oil relative to water), the more lipid soluble it is, and therefore, the faster its rate of movement across a bilayer. The first and rate-
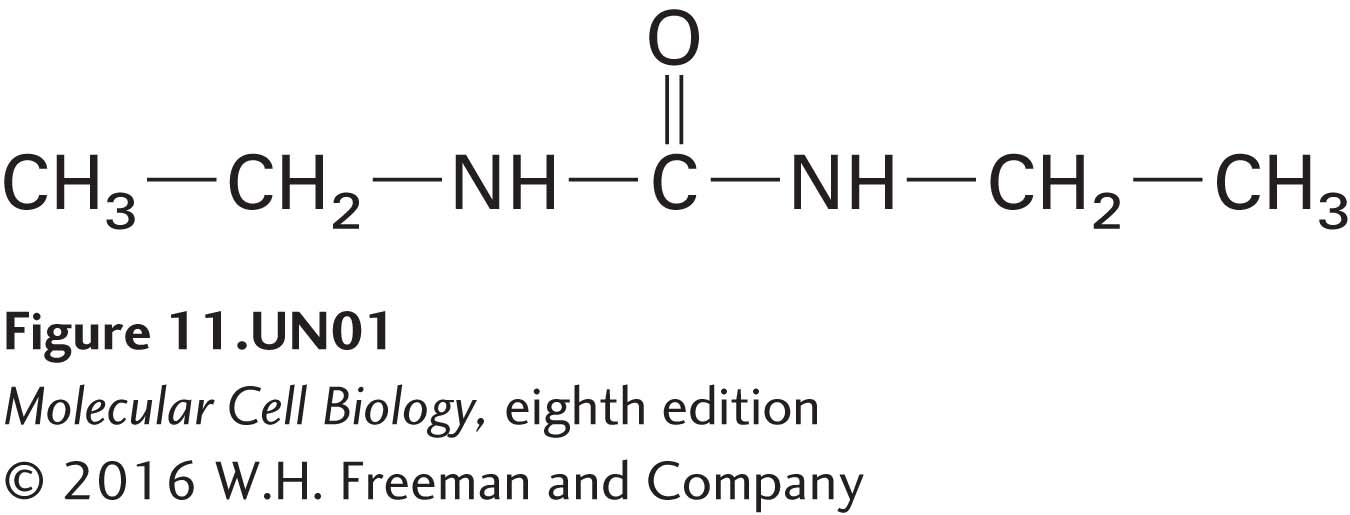
has a K of 0.01, whereas urea
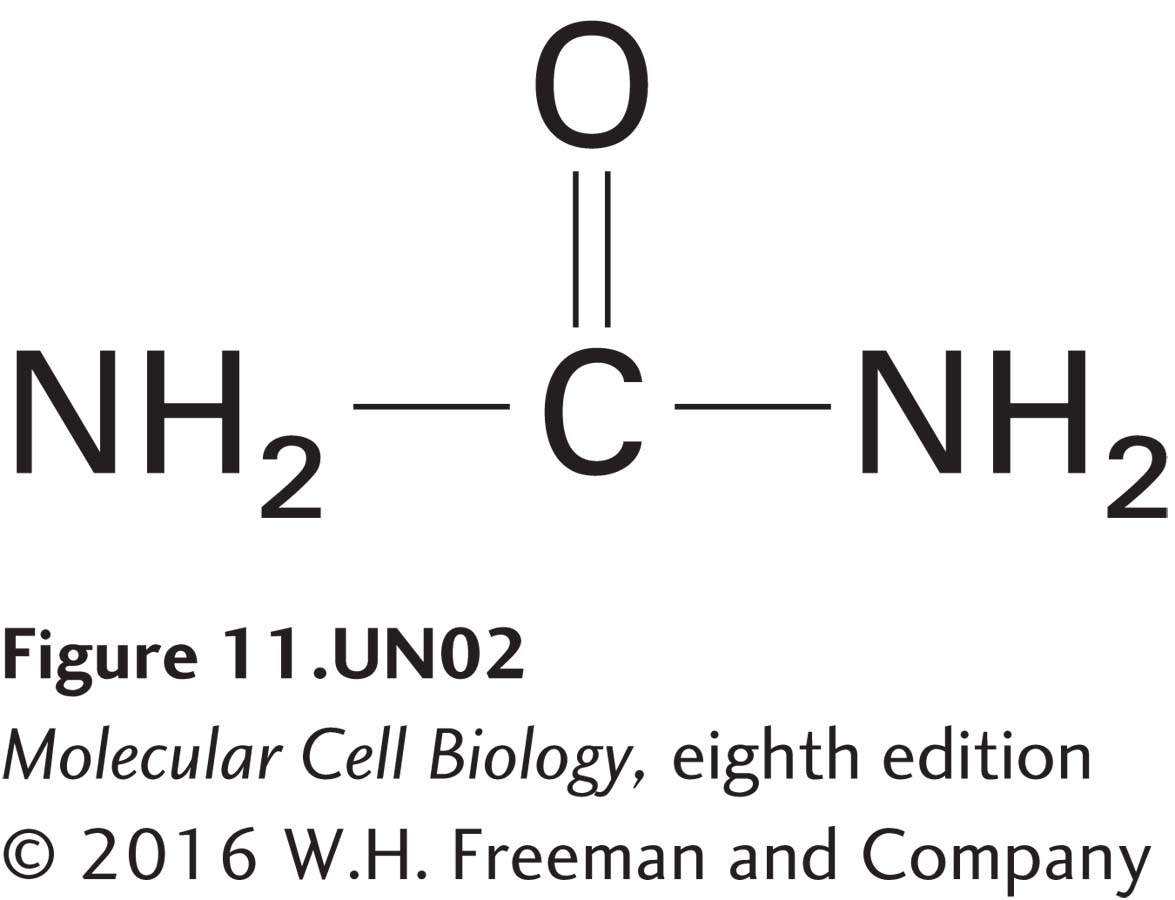
has a K of 0.0002. Diethylurea, which is 50 times (0.01/0.0002) more hydrophobic than urea, will therefore diffuse through a pure phospholipid bilayer about 50 times faster than urea. Similarly, fatty acids with longer hydrocarbon chains are more hydrophobic than those with shorter chains and at all concentrations will diffuse more rapidly across a pure phospholipid bilayer.
If a substance carries a net charge, its movement across a membrane is influenced by both its concentration gradient and the membrane potential, the electric potential (voltage) across the membrane. The combination of these two forces, called the electrochemical gradient, determines the energetically favorable direction of movement of a charged molecule across a membrane. The electric potential that exists across most cellular membranes results from a small imbalance in the concentrations of positively and negatively charged ions on the two sides of the membrane. We discuss how this ionic imbalance, and the resulting potential, arise and are maintained in Sections 11.4 and 11.5.