Mitochondrial Oxidation of Fatty Acids Generates ATP
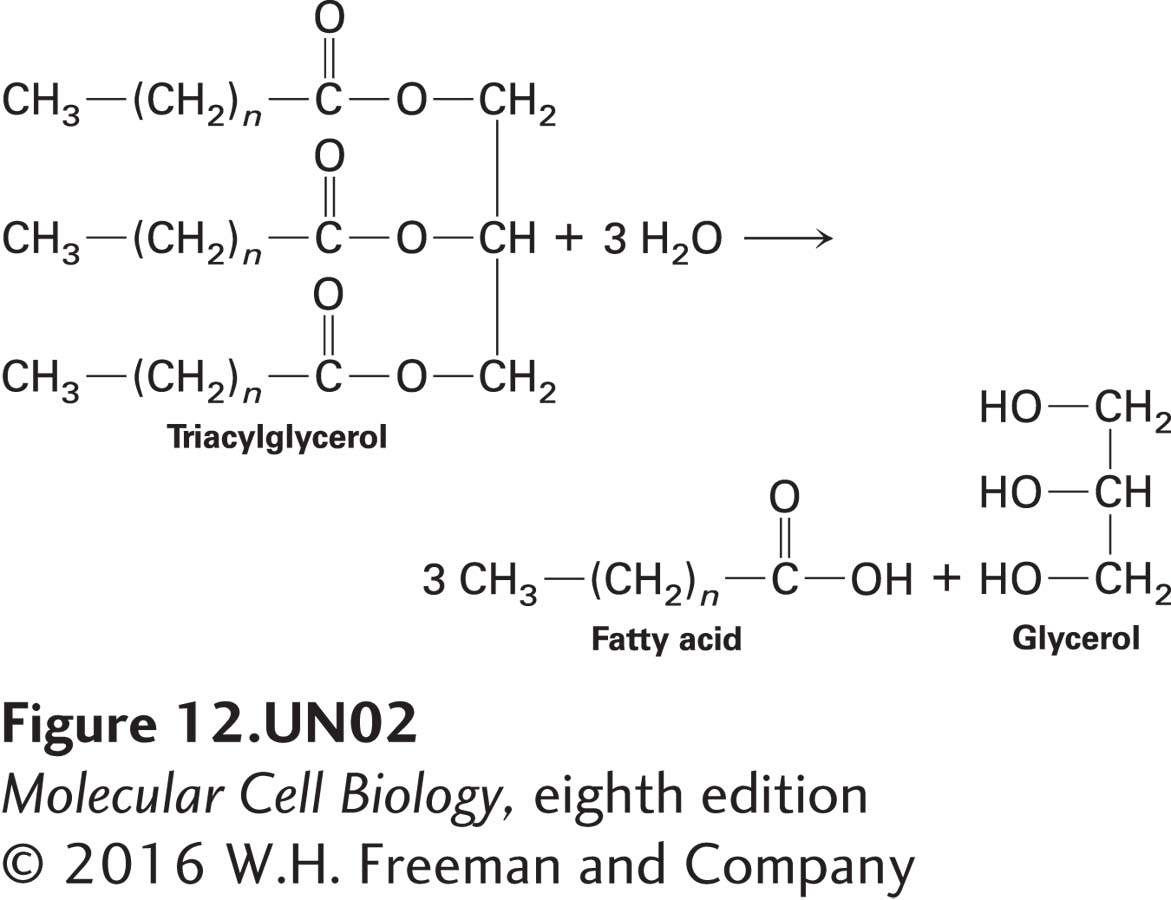
Up to now, we have focused mainly on the oxidation of carbohydrates, namely glucose, for ATP generation. Fatty acids are another important source of cellular energy. Cells can take up either glucose or fatty acids from the extracellular space with the help of specific transporter proteins (see Chapter 11). Should a cell not need to burn these molecules immediately, it can store them as a polymer of glucose called glycogen (especially in muscle or liver) or as a trimer of fatty acids covalently linked to glycerol, called a triacylglycerol or triglyceride (see below). In some cells, excess glucose is converted into fatty acids and then triacylglycerols for storage. However, unlike microorganisms, animals are unable to convert fatty acids to glucose. When the cells need to burn these energy stores to make ATP (e.g., when a resting muscle begins to do work and needs to burn glucose or fatty acids as fuel), enzymes break down glycogen to glucose or hydrolyze triacylglycerols to fatty acids, which are then oxidized to generate ATP:
Page 537
Fatty acids are the major energy source for some tissues, particularly adult heart muscle. In humans, in fact, more ATP is generated by the oxidation of fats than by the oxidation of glucose. The oxidation of 1 g of triacylglycerol to CO2 generates about six times as much ATP as does the oxidation of 1 g of hydrated glycogen. Thus, considering the mass of stored fuel an organism must carry, triglycerides are more efficient than carbohydrates for storage of energy, in part because they are stored in anhydrous form and can yield more energy when oxidized, and in part because they are intrinsically more reduced (have more hydrogens) than carbohydrates. In mammals, the primary site of storage of triacylglycerol is fat (adipose) tissue, whereas the primary sites for glycogen storage are muscle and the liver. In animals, when tissues need to generate a lot of ATP, as in exercising muscle, signals are sent to adipose tissue to hydrolyze triacylglycerols and to release the fatty acids into the circulatory system so that they can move to and be transported into the ATP-
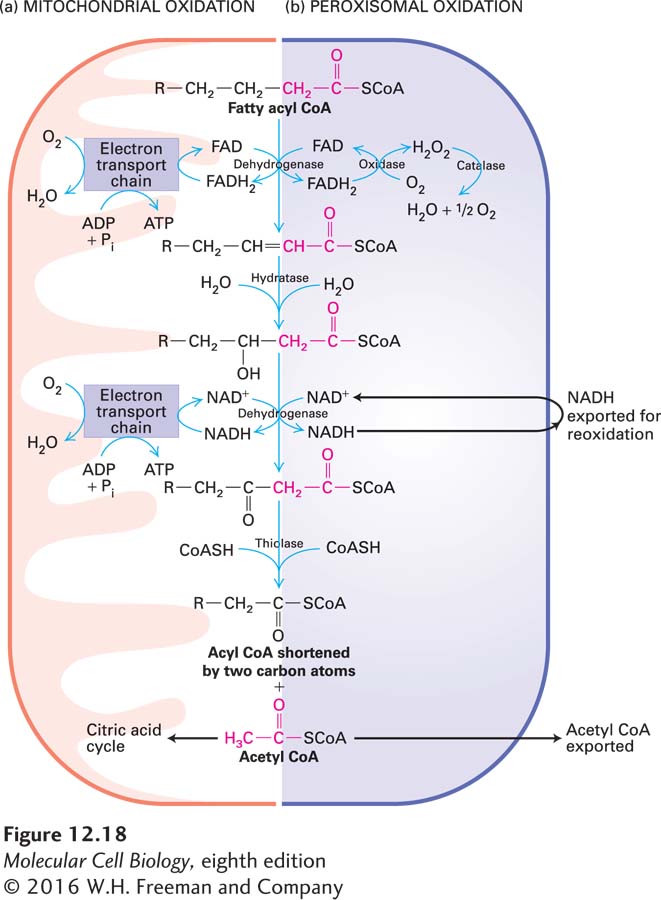
Just as there are four stages in the oxidation of glucose, there are four stages in the oxidation of fatty acids. To optimize the efficiency of ATP generation, part of stage II (citric acid cycle oxidation of acetyl CoA) and all of stages III and IV of fatty acid oxidation are identical to those of glucose oxidation. The differences lie in cytosolic stage I and in the first part of mitochondrial stage II. In stage I, fatty acids are converted to a fatty acyl CoA in the cytosol in a reaction coupled to the hydrolysis of ATP to AMP and PPi (inorganic pyrophosphate) (see Figure 12-14):
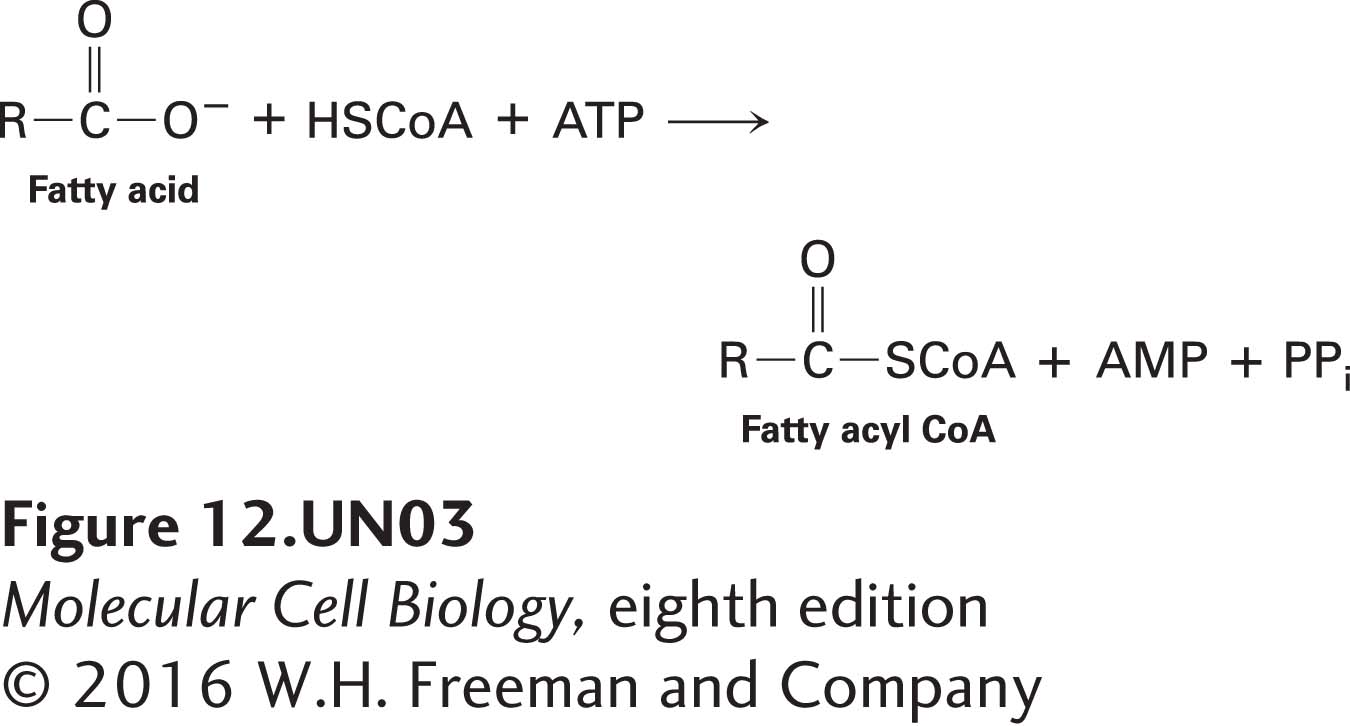
Subsequent hydrolysis of PPi to two molecules of Pi releases energy that drives this reaction to completion. To enter the mitochondrial matrix, the fatty acyl group must be covalently transferred to a molecule called carnitine and moved across the inner mitochondrial membrane by an acylcarnitine transporter protein (see Figure 12-14, blue oval); then, on the matrix side, the fatty acyl group is released from carnitine and reattached to another CoA molecule. The activity of the acylcarnitine transporter is regulated to prevent oxidation of fatty acids when cells have adequate energy (ATP) supplies.
In the first part of stage II, each molecule of a fatty acyl CoA in the mitochondrion is oxidized in a cyclical sequence of four reactions in which all the carbon atoms are converted, two at a time, to acetyl CoA with generation of FADH2 and NADH (Figure 12-18a). For example, mitochondrial oxidation of each molecule of the 18-