Chapter Introduction
CHAPTER 11: Transmembrane Transport of Ions and Small Molecules
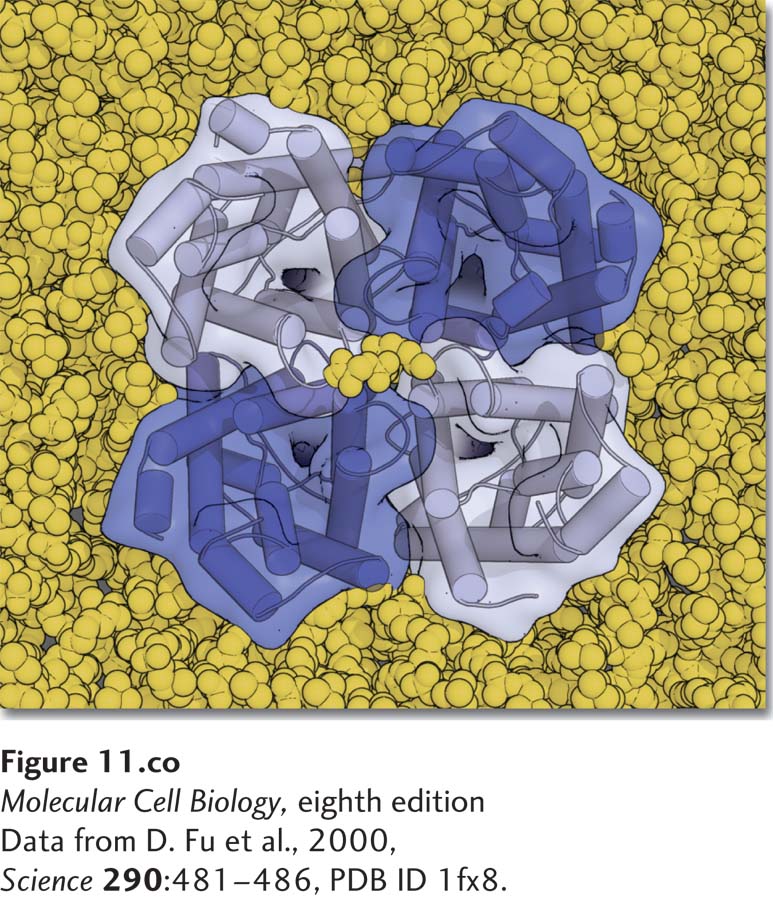
In all cells, the plasma membrane forms the barrier that separates the cytoplasm from the exterior environment, thus defining a cell’s physical and chemical boundaries. By preventing the unimpeded movement of molecules and ions into and out of the cell, the plasma membrane maintains essential differences between the composition of the extracellular fluid and that of the cytosol. For example, the concentration of sodium chloride (NaCl) in the blood and extracellular fluids of animals is generally above 150 mM, similar to the ~450 mM Na+ found in the seawater, in which all cells are thought to have evolved. In contrast, the sodium ion (Na+) concentration in the cytosol is tenfold lower, about 15 mM, while the potassium ion (K+) concentration is higher in the cytosol than outside.
Organelle membranes, which separate the cytosol from the interior of the organelle, also form permeability barriers. For example, the proton concentration in the lysosome interior, pH 5, is about a hundredfold greater than that of the cytosol, and many specific metabolites accumulate at higher concentrations in the interior of other organelles, such as the endoplasmic reticulum or the Golgi complex, than in the cytosol.
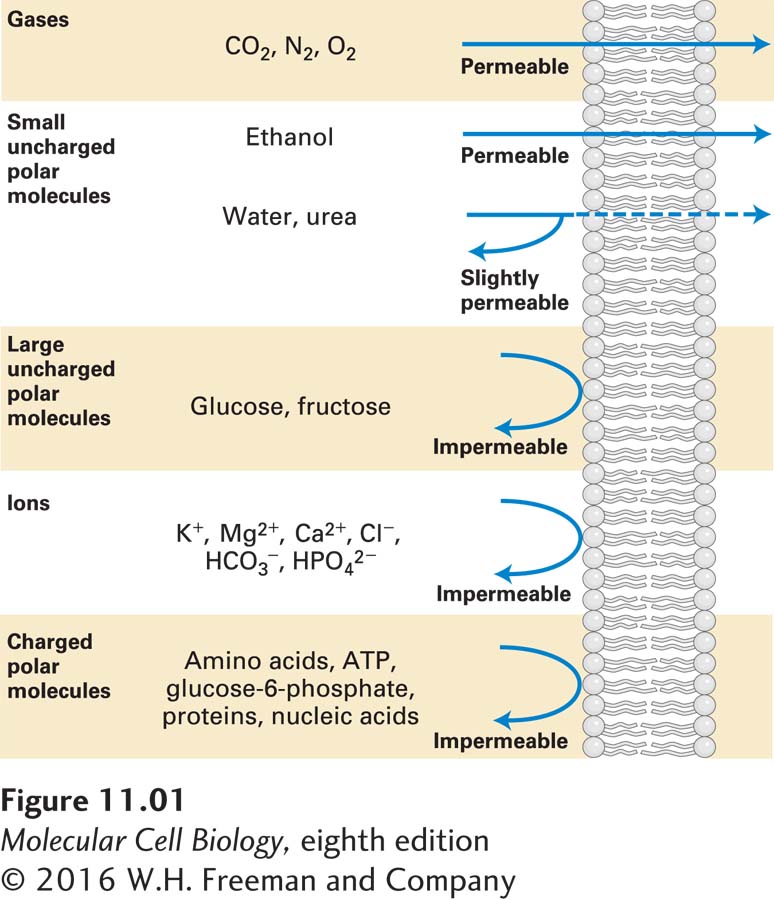
All cellular membranes, both plasma membranes and organelle membranes, consist of a bilayer of phospholipids in which other lipids and specific types of proteins are embedded. It is this combination of lipids and proteins that gives cellular membranes their distinctive permeability qualities. If cellular membranes were pure phospholipid bilayers (see Figure 10-4), they would be excellent chemical barriers, impermeable to virtually all ions, amino acids, sugars, and other water-
Page 474
Movement of virtually all small molecules and ions across cellular membranes is mediated by membrane transport proteins—integral membrane proteins with multiple transmembrane domains embedded in cellular membranes. These membrane-
We begin our discussion of membrane transport proteins by reviewing some of the general principles of transport across membranes and distinguishing between three major classes of such proteins. In subsequent sections, we describe the structure and operation of specific examples of each class and show how members of families of homologous transport proteins have different properties that enable different cell types to function appropriately. We also explain how specific combinations of transport proteins in both the plasma membrane and organelle membranes enable cells to carry out essential physiological processes, including the maintenance of cytosolic pH, the accumulation of sucrose and salts in plant cell vacuoles, and direction of the flow of water in both plants and animals. The cell’s resting membrane potential is an important consequence of selective ion transport across membranes, and we consider how this potential arises. Epithelial cells, such as those lining the small intestine, use a combination of membrane transport proteins to transport ions, sugars and other small molecules, and water from one side of the cell to the other. We will see how our understanding of this process has led to the development of sports drinks as well as therapies for cholera and other diarrheal diseases.
Note that in this chapter we cover only transport of small molecules and ions; transport of larger molecules, such as proteins and oligosaccharides, is covered in Chapters 13 and 14.