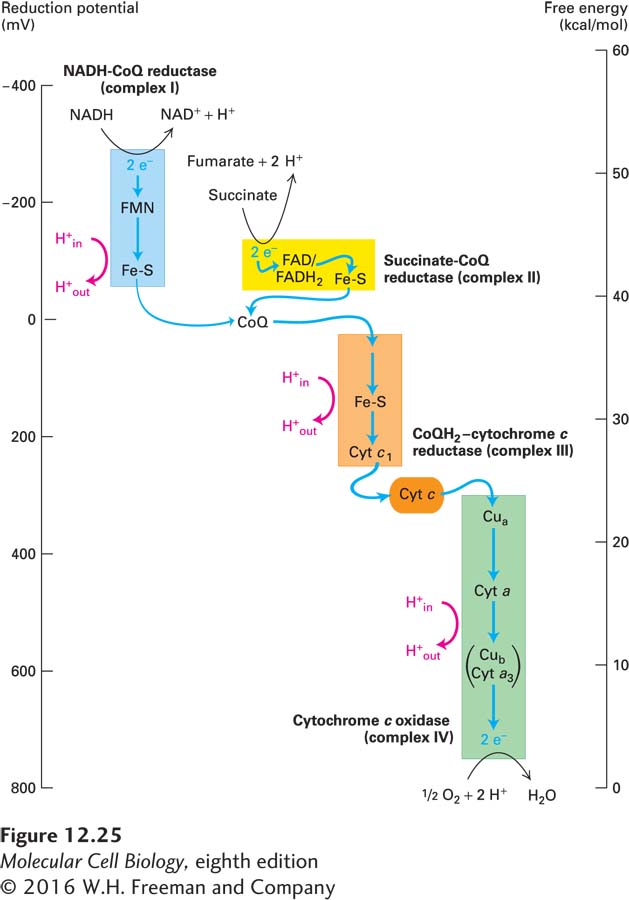
The Reduction Potentials of Electron Carriers in the Electron-Transport Chain Favor Electron Flow from NADH to O2
As we saw in Chapter 2, the reduction potential (E) for a partial reduction reaction
Oxidized molecule + e− ⇌ reduced molecule
is a measure of the equilibrium constant of that partial reaction. With the exception of the b cytochromes in complex III (CoQH2–cytochrome c reductase), the standard reduction potential E°′ of the electron carriers in the electron-
NAD+ + H+ + 2 e− ⇌ NADH
the value of the standard reduction potential is −320 mV, which is equivalent to a ΔG°′ of +14.8 kcal/mol for transfer of two electrons. Thus this partial reaction tends to proceed toward the left; that is, toward the oxidation of NADH to NAD+.
In contrast, the standard reduction potential for the partial reaction
Cytochrome cox (Fe3+) + e− ⇌ cytochrome cred (Fe2+)
is +220 mV (ΔG°′ = −5.1 kcal/mol) for transfer of one electron. Thus this partial reaction tends to proceed toward the right; that is, toward the reduction of cytochrome c (Fe3+) to cytochrome c (Fe2+).
The final reaction in the electron-
2 H+ + ½ O2 + 2 e− → H2O
has a standard reduction potential of +816 mV (ΔG°′ = −37.8 kcal/mol for transfer of two electrons), the most positive in the whole series; thus this reaction also tends to proceed toward the right.
As illustrated in Figure 12-25, the steady increase in E°′ values, and the corresponding decrease in ΔG°′ values, of the carriers in the electron-