The Mechanism of ATP Synthesis Is Shared Among Bacteria, Mitochondria, and Chloroplasts
Although bacteria lack internal membranes, aerobic bacteria nonetheless carry out oxidative phosphorylation by the same processes that occur in eukaryotic mitochondria and chloroplasts (Figure 12-30). Enzymes that catalyze the reactions of both the glycolytic pathway and the citric acid cycle are present in the cytosol of bacteria; enzymes that oxidize NADH to NAD+ and transfer the electrons to the ultimate acceptor O2 reside in the bacterial plasma membrane. The movement of electrons through these membrane carriers is coupled to the pumping of protons out of the cell. The movement of protons back into the cell, down their concentration gradient through ATP synthase, drives the synthesis of ATP. The bacterial ATP synthase (F0F1 complex) is essentially identical in structure and function to the mitochondrial and chloroplast ATP synthases, but is simpler to purify and study.
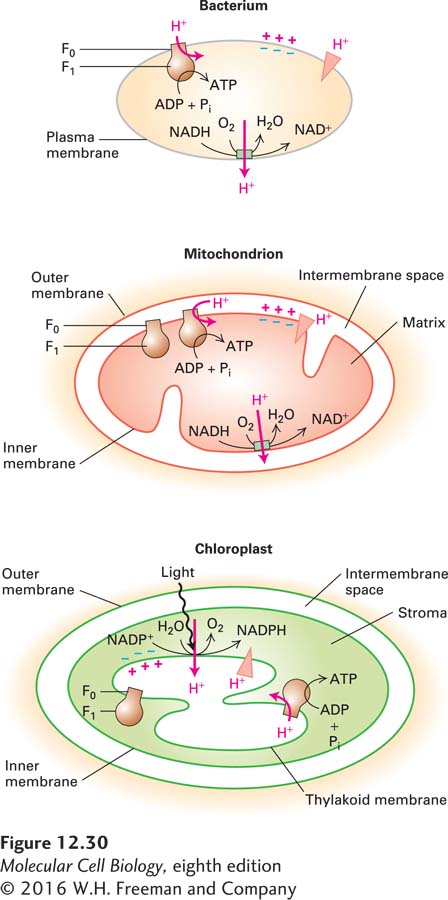
Why is the mechanism of ATP synthesis shared among both prokaryotic organisms and eukaryotic organelles? Primitive aerobic bacteria were probably the progenitors of both mitochondria and chloroplasts in eukaryotic cells (see Figure 12-7). According to this endosymbiont hypothesis, the inner mitochondrial membrane was derived from the bacterial plasma membrane, with its cytosolic face pointing toward what became the matrix of the mitochondrion. Similarly, in plants, the progenitor bacterium’s plasma membrane became the chloroplast’s thylakoid membrane, and its cytosolic face pointed toward what became the stromal space of the chloroplast (chloroplast structure will be described in Section 12.6). In all cases, ATP synthase is positioned with the globular F1 domain, which catalyzes ATP synthesis, on the cytosolic face of the membrane, so ATP is always formed on the cytosolic face (see Figure 12-30). Protons always flow through ATP synthase from the exoplasmic to the cytosolic face of the membrane. This flow is driven by the proton-
In addition to ATP synthesis, the proton-
Page 553