17.1 Microfilaments and Actin Structures
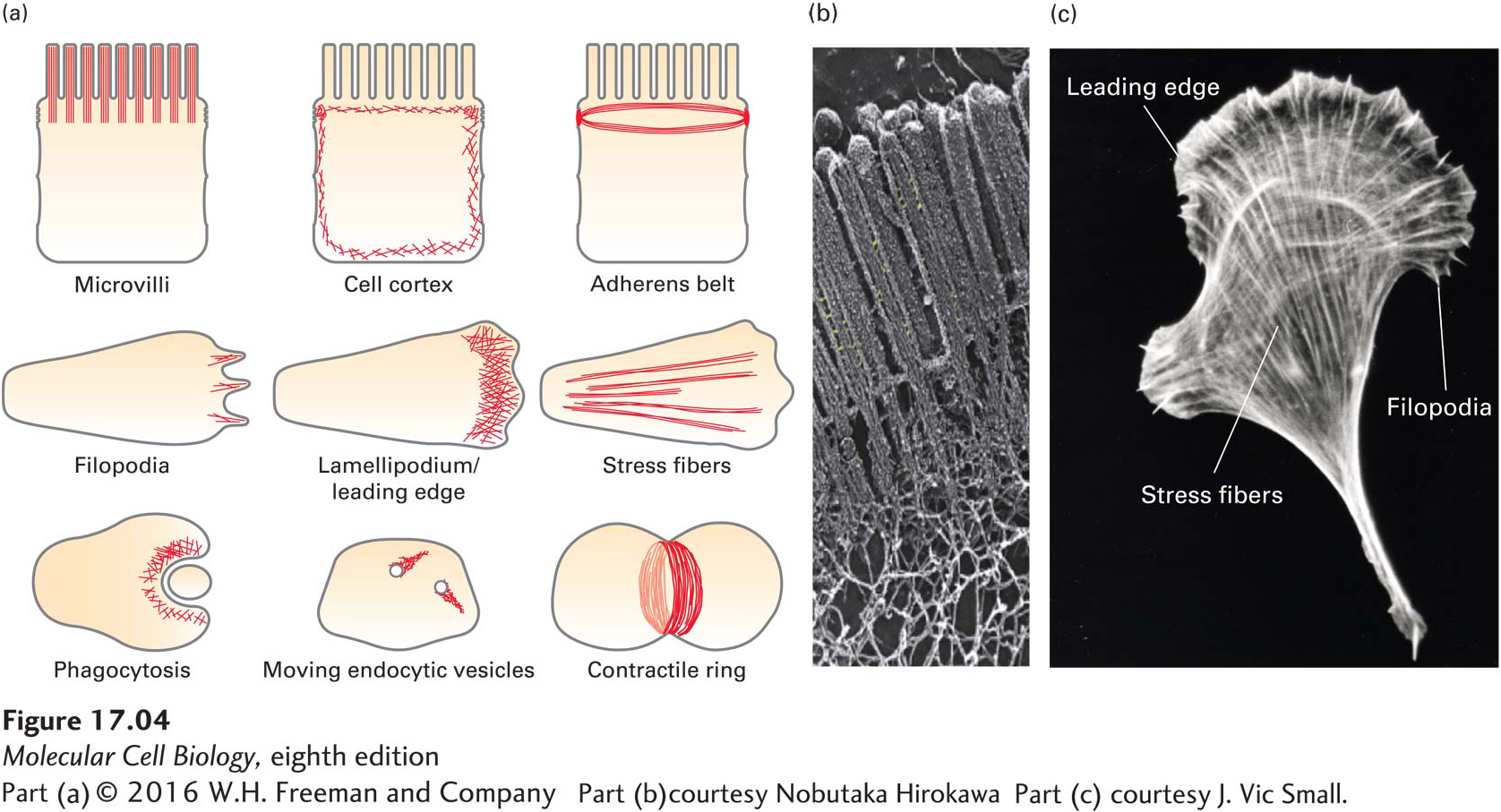
Microfilaments can assemble into a wide variety of different types of structures within a cell (Figure 17-4a). Each of these diverse structures underlies particular cellular functions. Microfilaments can exist as a tight bundle of filaments making up the core of the slender, fingerlike microvilli, but they can also be found in a less ordered network beneath the plasma membrane, known as the cell cortex, where they provide support and organization. In epithelial cells, microfilaments form a contractile band around the cell, the adherens belt, that is intimately associated with adherens junctions (see Chapter 20) to provide strength to the epithelium. In migrating cells, a network of microfilaments is found at the front of the cell in the leading edge, or lamellipodium, from which bundles of filaments called filopodia may protrude. Many cells have contractile microfilaments called stress fibers, which attach to the external substratum as cells migrate (discussed in Chapter 20). Specialized cells such as macrophages use contractile microfilaments to engulf and internalize pathogens, which are then destroyed internally. Highly dynamic, short bursts of actin filament assembly can power the movement of endocytic vesicles away from the plasma membrane. At a late stage of cell division in animals, after all the organelles have been duplicated and segregated, a contractile ring forms and constricts to generate two daughter cells in a process known as cytokinesis. Thus cells use actin filaments in many ways: in a structural role, by harnessing the power of actin polymerization to do work, or as tracks for myosin motors. The electron micrograph in Figure 17-4b shows microfilaments in microvilli. Different arrangements of microfilaments often coexist within a single cell, as shown in Figure 17-4c for a migrating fibroblast.
The basic building block of microfilaments is actin, a protein that has the remarkable capacity for reversibly assembling into a polarized filament with functionally distinct ends. These filaments are then molded into the various structures described in the previous paragraph by actin-