Integrins Mediate Cell-ECM Adhesions, Including Those in Epithelial-Cell Hemidesmosomes
To be stably anchored to solid tissues and organs, simple columnar epithelial sheets must be firmly attached via their basal surfaces to the underlying ECM (basal lamina). This attachment occurs via adhesion receptors called integrins (see Figure 20-2), which are located both within and outside of anchoring junctions called hemidesmosomes (see Figure 20-11a). Hemidesmosomes comprise integral membrane proteins linked via cytoplasmic adapter proteins (e.g., plakins) to keratin-based intermediate filaments. The principal ECM adhesion receptor in epithelial hemidesmosomes is integrin α6β4.
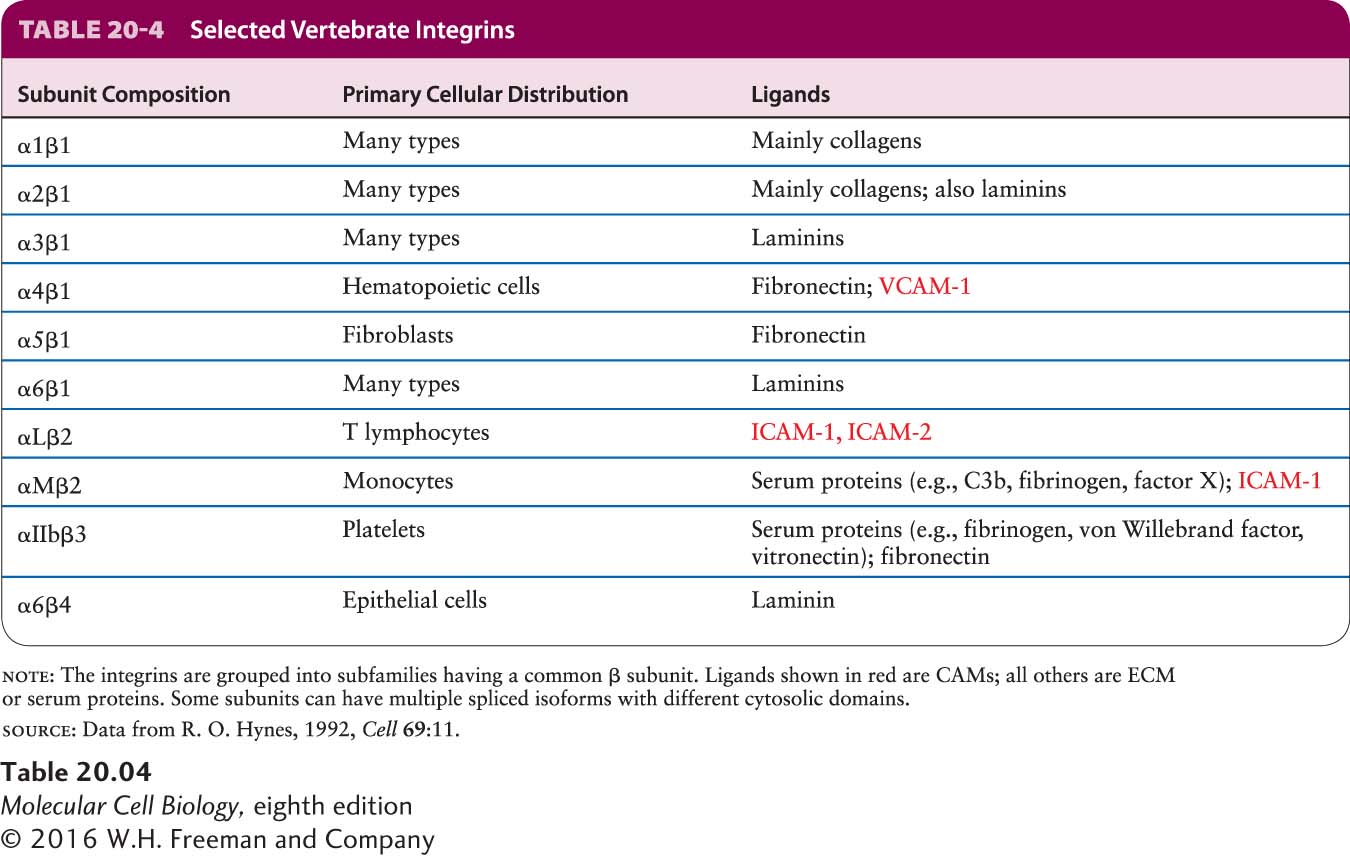
Integrins function as adhesion receptors and CAMs in a wide variety of epithelial and nonepithelial cells, mediating many cell-matrix and cell-cell interactions (Table 20-4). In vertebrates, at least 24 integrin heterodimers, composed of 18 types of α subunits and 8 types of β subunits in various αβ heterodimeric combinations, are known. A single type of β chain can interact with any one of several different types of α chains, forming distinct integrins that bind different ligands. This phenomenon of combinatorial diversity allows a relatively small number of components to serve a large number of distinct functions. Although most cells express several distinct integrins that bind the same or different ligands, many integrins are expressed predominantly in certain types of cells. Not only do many integrins bind more than one ligand, but there are ligands that can bind to any one of several different integrins.
All integrins appear to have evolved from two ancient general subgroups: those that bind proteins containing the tripeptide sequence Arg-Gly-Asp, usually called the RGD motif (fibronectin is one such protein), and those that bind laminin. Several integrin α subunits contain a distinctive inserted domain, the I-domain, which can mediate binding of certain integrins to various collagens in the ECM. Some integrins with I-domains are expressed exclusively on leukocytes (white blood cells) and red and white blood cell precursor (hematopoietic) cells. I-domains also recognize CAMs on other cells, including members of the Ig superfamily (e.g., ICAMs, VCAMs), and thus participate in cell-cell adhesion.
Integrins typically exhibit low affinities for their ligands, with dissociation constants (Kd) between 10−6 and 10−7 mol/L. However, the multiple weak interactions generated by the binding of hundreds or thousands of integrin molecules to their ligands on cells or in the ECM allow a cell to remain firmly anchored to its ligand-expressing target.
Page 939
Parts of both the α subunit and the β subunit of an integrin molecule contribute to the primary extracellular ligand-binding site (see Figure 20-2). Ligand binding to integrins also requires the simultaneous binding of divalent cations. Like that of other adhesion molecules, the cytosolic region of integrins interacts with adapter proteins, which in turn bind to the cytoskeleton and to intracellular signaling molecules (see Figure 20-8). Most integrins are linked via adapters to the actin cytoskeleton, including two of the integrins that connect the basal surface of epithelial cells to the basal lamina via the ECM molecule laminin. Some integrins, however, interact with intermediate filaments. The cytosolic domain of the β4 chain in the α6β4 integrin in hemidesmosomes (see Figure 20-1), which is much longer than the cytosolic domains of other integrin β chains, binds to specialized adapter proteins, which in turn interact with keratin-based intermediate filaments (see Table 20-4). Other integrins (for example, α3β1) are the adhesion receptors in the focal contacts linking the epithelial basal lamina with the actin cytoskeleton (see Figure 20-1).
As we will see, the diversity of integrins and their ECM ligands allows integrins to participate in a wide array of key biological processes, including the inflammatory response and the migration of cells to their correct locations during morphogenesis. The importance of integrins in diverse processes is highlighted by the defects exhibited by knockout mice engineered to have mutations in various integrin subunit genes. These defects include major abnormalities in development, blood vessel formation, leukocyte function, inflammation, bone remodeling, and blood clotting. Despite their differences, all these processes depend on integrin-mediated interactions between the cytoskeleton and either the ECM or CAMs on other cells.
In addition to their adhesion function, integrins can mediate outside-in and inside-out signaling (see Figure 20-8). The engagement of integrins by their extracellular ligands can, through adapter proteins bound to the integrin’s cytosolic region, influence the cytoskeleton and intracellular signaling pathways (outside-in signaling). Conversely, intracellular signaling pathways can alter the structure of integrins and consequently their abilities to adhere to their extracellular ligands and mediate cell-cell and cell-ECM interactions (inside-out signaling). Integrin-mediated signaling pathways influence processes as diverse as cell survival, cell proliferation, and programmed cell death (see Chapter 21).