Molecular Complementarity Due to Noncovalent Interactions Leads to a Lock-and-Key Fit Between Biomolecules
Both inside and outside cells, ions and molecules constantly collide. The higher the concentration of any two types of molecules, the more likely they are to encounter each other. When two molecules encounter each other, they are most likely to simply bounce apart because the noncovalent interactions that would bind them together are weak and have a transient existence at physiological temperatures. However, molecules that exhibit molecular complementarity, a lock-
Figure 2-12 illustrates how multiple, different weak interactions can cause two hypothetical proteins to bind together tightly. Numerous examples of such protein-
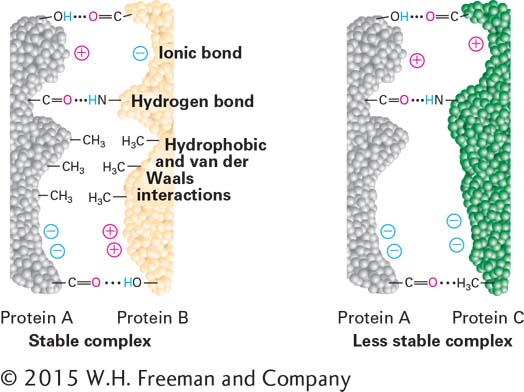
Depending on the number and strength of the noncovalent interactions between the two molecules and on their environment, their binding may be tight or loose and, as a consequence, either lasting or transient. The higher the affinity of two molecules for each other, the better the molecular “fit” between them, the more noncovalent interactions can form, and the more tightly they can bind together. An important quantitative measure of affinity is the binding dissociation constant Kd, described in Section 2.3. It is important to note that many large biological molecules are not hard, rigid structures, but rather can be somewhat malleable. Thus the binding of a molecule to another has the potential to induce a change in the shape of its binding partner. When the molecular complementarity increases after such interactions, the process is called induced fit.
As we discuss in Chapter 3, nearly all the chemical reactions that occur in cells also depend on the binding properties of enzymes. These proteins not only speed up, or catalyze, reactions, but do so with a high degree of specificity, which is a reflection of their ability to bind tightly to only one or a few related molecules. The specificity of intermolecular interactions and reactions, which depends on molecular complementarity, is essential for many processes critical to life.