Liquid Chromatography Resolves Proteins by Mass, Charge, or Affinity
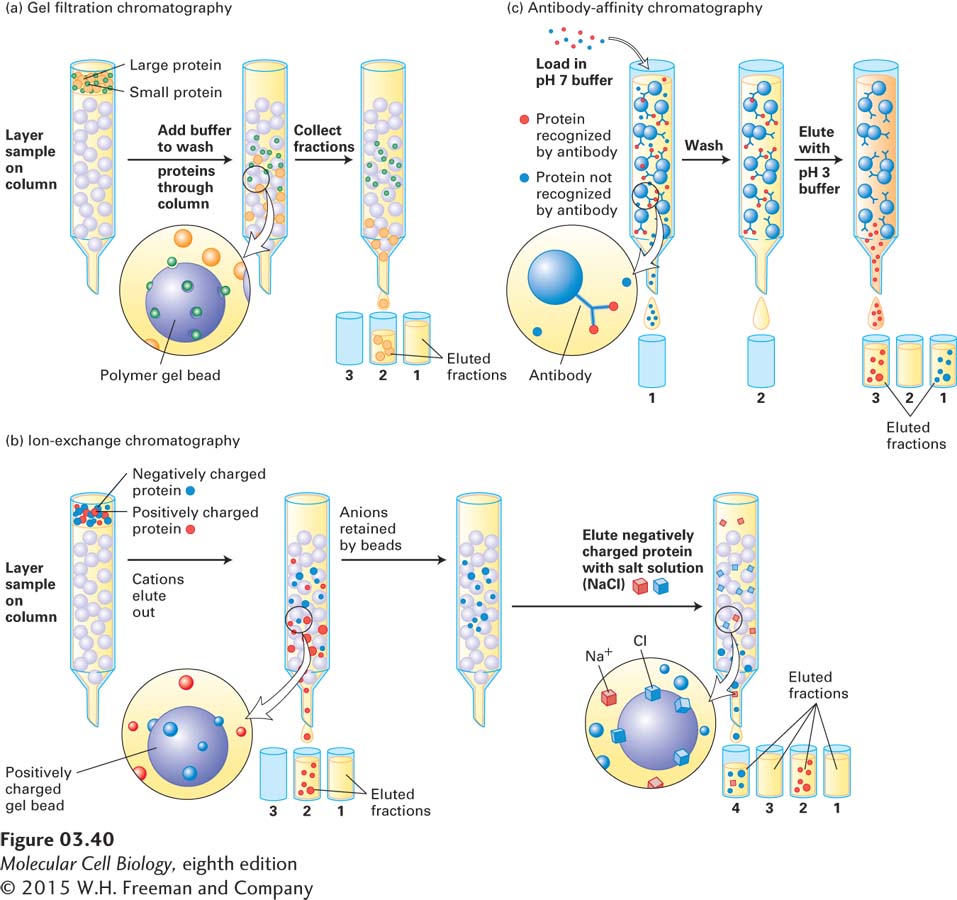
A third common technique for separating mixtures of proteins or fragments of proteins, as well as other molecules, is based on the principle that molecules in solution can differentially interact with (bind to and dissociate from) a particular solid surface, depending on the physical and chemical properties of the molecule and the surface. If the solution is allowed to flow across the surface, then molecules that interact frequently with the surface will spend more time bound to the surface, and thus flow past the surface more slowly, than molecules that interact infrequently with it. In this technique, called liquid chromatography (LC), the sample is placed on top of a tightly packed column of spherical beads held within a glass, metal, or plastic cylinder (Figure 3-40). The sample then flows down the column, driven by gravitational or hydrostatic forces alone or sometimes with the assistance of a pump. In some LC systems, the composition of the fluid flowing out of the column is monitored continuously (for example, by spectroscopy). Small aliquots of fluid flowing out of the column, called fractions, are collected sequentially and can be analyzed subsequently for their contents and chemical activities (e.g., enzymatic activity). The nature of the beads in the column determines whether the separation of proteins depends on differences in their mass, charge, or other binding properties (e.g., affinity for substances attached to the beads).
Page 110
Page 111
Gel Filtration Chromatography Proteins that differ in mass can be separated on a column of porous beads made from polyacrylamide, dextran (a bacterial polysaccharide), or agarose (a seaweed derivative)—a technique called gel filtration chromatography. Although proteins flow around the beads, they spend some time within the large depressions that cover a bead’s surface. Because smaller proteins can penetrate these depressions more readily than larger proteins can, they travel through a gel filtration column more slowly than larger proteins do (Figure 3-40a). (In contrast, proteins migrate through the pores in an electrophoretic gel; thus smaller proteins move faster than larger ones.) The total volume of liquid required to elute (or separate and remove) a protein from a gel filtration column depends on the protein’s mass: the smaller its mass, the longer it is trapped on the beads, the longer it takes to traverse the column, and the greater the elution volume. If proteins of known mass are used as standards to calibrate the column, the elution volume can be used to estimate the mass of a protein in a mixture. A protein’s shape as well as its mass can influence the elution volume.
Ion-
The proteins in a mixture carry various net charges at any given pH. When a solution of mixed proteins flows through a column of positively charged beads, only proteins with a net negative charge (acidic proteins) adhere to the beads; neutral and positively charged (basic) proteins flow unimpeded through the column (Figure 3-40b). The acidic proteins are then eluted selectively from the column by passing a solution of increasing concentrations of salt (a salt gradient) through the column. At low salt concentrations, protein molecules and beads are attracted by their opposite charges. At higher salt concentrations, negatively charged salt ions bind to the positively charged beads, displacing the negatively charged proteins. In a gradient of increasing salt concentrations, weakly bound proteins—
Affinity Chromatography The ability of proteins to bind specifically to other molecules is the basis of affinity chromatography. In this technique, ligands or other molecules that bind to the protein of interest are covalently attached to the beads used to form the column. Ligands can be enzyme substrates, inhibitors or their analogs, or other small molecules that bind to specific proteins. In a widely used form of this technique—
In principle, an affinity column will retain only those proteins that bind the molecule attached to the beads; the remaining proteins, regardless of their charges or masses, will pass through the column because they do not bind. However, if a retained protein is in turn bound to other molecules, forming a complex, then the entire complex is retained on the column. The proteins bound to the affinity column are then eluted by adding an excess of a soluble form of the ligand, by exposure of bound materials to detergents, or by changing the salt concentration or pH such that the binding to the molecule on the column is disrupted. The ability of this technique to separate particular proteins depends on the selection of appropriate binding partners that bind more tightly to the protein of interest than to other proteins.