CONCEPT2.5 Biochemical Changes Involve Energy
A chemical reaction occurs when atoms have sufficient energy to combine, or to change their bonding partners. Consider the hydrolysis of the disaccharide sucrose to its component monomers, glucose and fructose (see p. 27 for the chemical structures). We can express this reaction using a chemical equation:
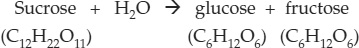
In this equation, sucrose and water are the reactants, and glucose and fructose are the products. The reaction proceeds as some bonds in the reactants are broken and new bonds form to make the products. Electrons and protons are transferred from one reactant to the other to form the products. The products of this reaction have different properties from those of the reactants. Chemical reactions involve changes in energy; for example, the energy contained in the chemical bonds of sucrose and water (the reactants) is greater than the energy in the bonds of the two products, glucose and fructose.
What is energy? Physicists define it as the capacity to do work, which occurs when a force operates on an object over a distance. But in biochemistry, it is more useful to consider energy as the capacity for change. In biochemical reactions, energy changes are usually associated with changes in the chemical composition and properties of molecules. Energy comes in many forms: chemical, electrical, heat, light, and mechanical. But all forms of energy can be considered as one of two basic types:
- Potential energy is the energy of state or position—that is, stored energy. It can be stored in many forms: in chemical bonds, as a concentration gradient, or even as an electric charge imbalance.
- Kinetic energy is the energy of movement—that is, the type of energy that does work, that makes things change. For example, heat causes molecular motions and can even break chemical bonds.
Potential energy can be converted into kinetic energy and vice versa, and the form that the energy takes can be converted. Think of reading this book: light energy is converted to chemical energy in your eyes, and then is converted to electrical energy in the nerve cells that carry messages to your brain. When you decide to turn a page, the electrical and chemical energy of nerves and muscles are converted to mechanical energy for movement of your hand and arm.
32
Metabolism involves reactions that store and release energy
The sum total of all the chemical reactions occurring in a biological system at a given time is called metabolism. Metabolic reactions involve energy changes, in which energy is either stored in, or released from, chemical bonds. In general, the formation of a bond releases energy, whereas the breaking of a bond requires an input of energy. More energy is released in the formation of a stronger, more stable (lower energy) bond than in the formation of a less stable (higher energy) one. Conversely, the breaking of a stronger bond consumes more energy than the breaking of a weaker bond. A chemical reaction will occur spontaneously if the total energy consumed by breaking bonds in the reactants is less than the total energy released by forming bonds in the products.
Anabolic reactions (collectively called anabolism) link simple molecules to form more complex molecules. Anabolic reactions require an input of energy because strong bonds within the smaller molecules must be broken to form the more complex molecules. For example, the formation of sucrose requires the breaking of strong O—H bonds in glucose and fructose. Reactions that require an input of energy are called endergonic or endothermic (FIGURE 2.14A). The energy used in anabolic reactions is stored in the newly formed (higher energy) chemical bonds.
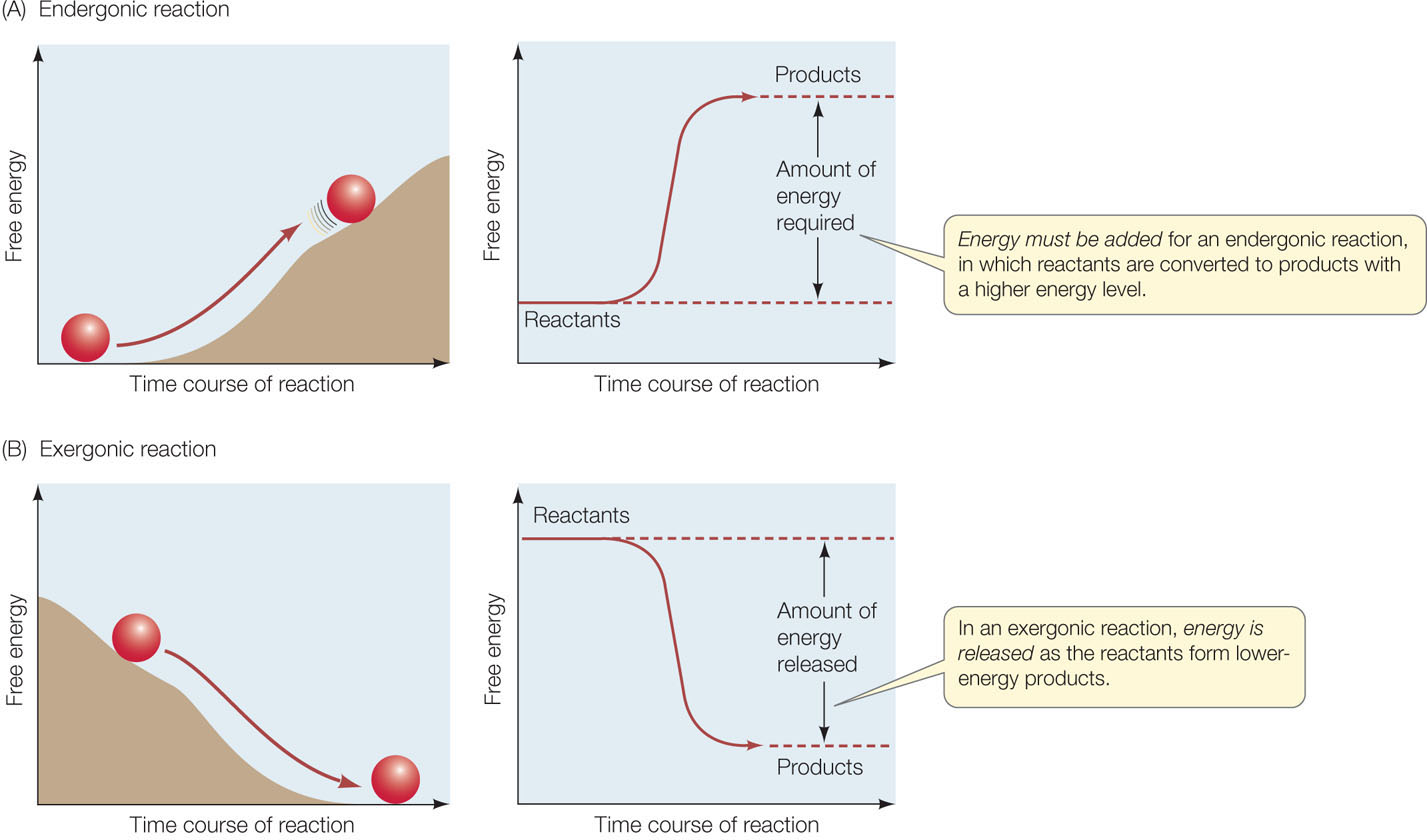
Catabolic reactions (collectively called catabolism) break down complex molecules into simpler ones and release the energy that was used to make the complex molecules. Chemists call such reactions exergonic or exothermic (FIGURE 2.14B). For example, when sucrose is hydrolyzed, energy is released by the formation of more stable (lower energy) bonds within the monosaccharides.
Catabolic and anabolic reactions are often linked. The energy released in catabolic reactions is often used to drive anabolic reactions—that is, to do biological work. For example, the energy released by the breakdown of glucose (catabolism) is used to drive anabolic reactions such as the synthesis of triglycerides. That is why fat accumulates if you eat food in excess of your energy needs.
Biochemical changes obey physical laws
Recall from the opening of this chapter that we described the mechanistic view of life, whereby living systems obey the same rules that govern the nonliving world. The laws of thermodynamics (thermo, “energy”; dynamics, “change”) were derived from studies of the fundamental properties of energy, and the ways energy interacts with matter. These laws apply to all matter and all energy transformations in the universe. Their application to living systems helps us understand how organisms and cells harvest and transform energy to sustain life.
33
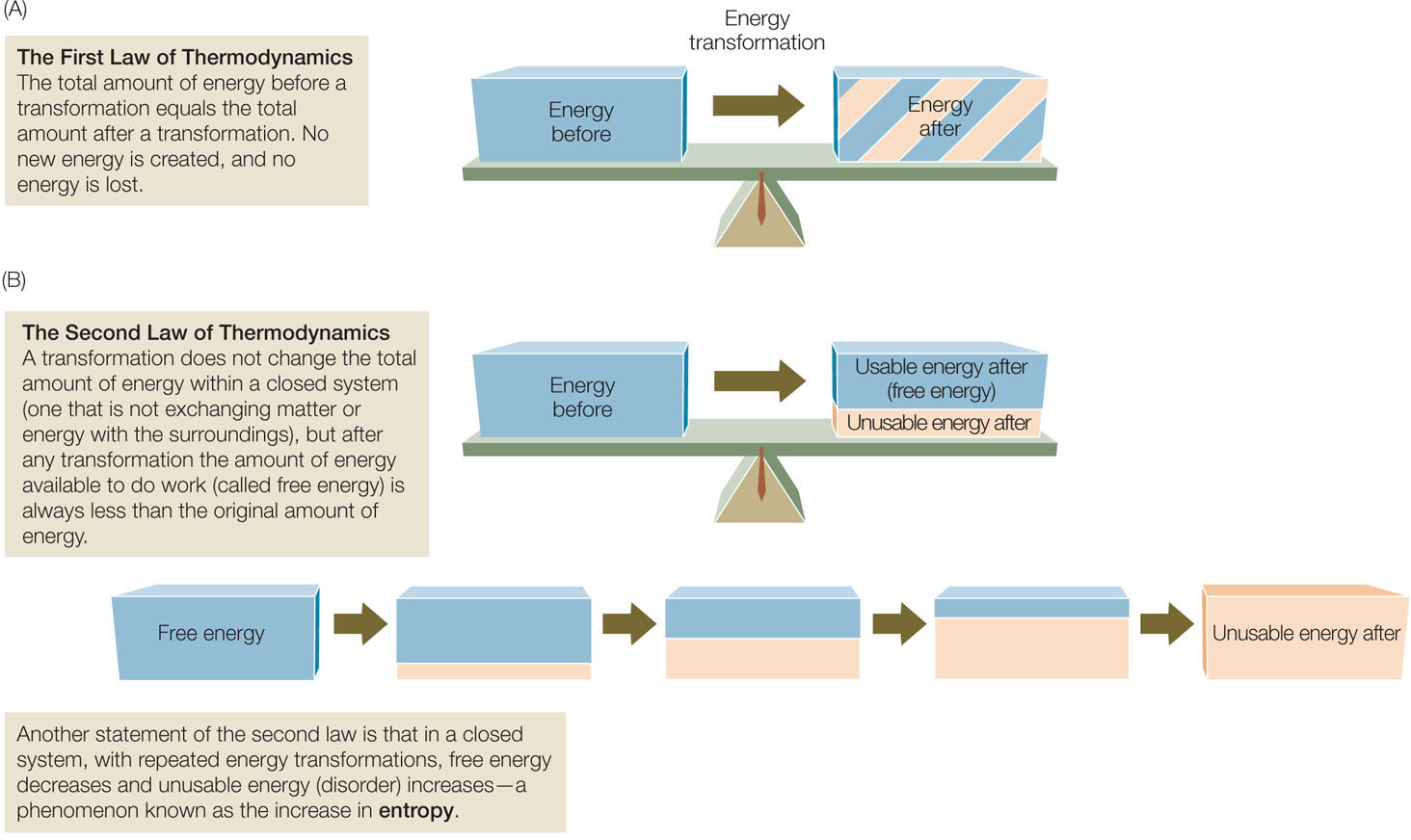
The first law of thermodynamics: Energy is neither created nor destroyed. The first law of thermodynamics states that in any conversion, energy is neither created nor destroyed. Another way of stating this is that the total energy before and after an energy conversion is the same (FIGURE 2.15A). [Similarly, matter is also conserved: in the hydrolysis of sucrose (see p. 31), there are 12 carbons, 24 hydrogens, and 12 oxygens on both sides of the equation.]
Although the total amount of energy is conserved, chemical reactions involve changes in the amount of (potential) energy stored in chemical bonds. If energy is released during the reaction, it is available to do work—for example, to drive another chemical reaction. In general, reactions that release energy (catabolic, or exergonic reactions) can occur spontaneously.
The second law of thermodynamics: Useful energy tends to decrease. Although energy cannot be created or destroyed, the second law of thermodynamics implies that when energy is converted from one form to another, some of that energy becomes unavailable for doing work (FIGURE 2.15B). In other words, no physical process or chemical reaction is 100 percent efficient; some of the released energy is lost in a form associated with disorder. Think of disorder as a kind of randomness caused by the thermal motion of particles; this energy is so dispersed that it is unusable. Entropy is a measure of the disorder in a system.
If a chemical reaction increases entropy, its products are more disordered or random than its reactants. The disorder in a solution of glucose and fructose is greater than that in a solution of sucrose, where the glycosidic bond between the two monosaccharides prevents free movement. Conversely, if there are fewer products and they are more restrained in their movements than the reactants, the disorder is reduced. But this requires an energy input to achieve.
The second law of thermodynamics predicts that, as a result of energy transformations, disorder tends to increase; some energy is always lost to random thermal motion (entropy). Chemical changes, physical changes, and biological processes all tend to increase entropy (see Figure 2.15B), and this tendency gives direction to these processes. Changes in entropy are mathematically related to changes in free energy (the energy available to do work), and thus the second law helps explain why some reactions proceed in one direction rather than another.
How does the second law of thermodynamics apply to organisms? Consider the human body, with its highly organized tissues and organs composed of large, complex molecules. This level of complexity appears to be in conflict with the second law, but for two reasons, it is not. First, the construction of complex molecules also generates disorder. The anabolic reactions needed to construct 1 kg of an animal body require the catabolism of about 10 kg of food. So metabolism creates far more disorder (more energy is lost to entropy) than the amount of order stored in flesh. Second, life requires a constant input of energy to maintain order. Without this energy, the complex structures of living systems would break down. Because energy is used to generate and maintain order, and biological processes cause an overall increase in entropy, there is no conflict with the second law of thermodynamics.
34
CHECKpointCONCEPT2.5
- When you eat a candy bar and then decide to go for a walk, energy transformations take place. Beginning with the food energy in the candy bar, describe the forms of energy used and the changes in energy that occur as you decide to walk and as you do the walking.
- What is the difference between anabolism and catabolism? Between endergonic and exergonic reactions?
- Predict whether these situations are endergonic or exergonic, and explain your reasoning:
- The formation of a phospholipid bilayer membrane
- Turning on a TV set
Biochemical changes involve energy
Chemical reactions in living systems involve changes in energy. These can be expressed as changes in available energy, called free energy (designated G, for Gibbs—the scientist who first described this parameter). The overall direction of a spontaneous chemical reaction is from higher to lower free energy. In other words, if the Greactants is greater than the Gproducts (negative ΔG; the Greek letter delta stands for “change in” or “difference”), the reaction will be spontaneous; it will tend to go in the direction from reactants to product and release free energy in the process. Reactions where the Greactants is less than the Gproducts (positive ΔG) will occur only if additional free energy is supplied.
The table shows some reactions and the absolute values of their associated free energy changes, |ΔG| (the vertical lines indicate absolute value).
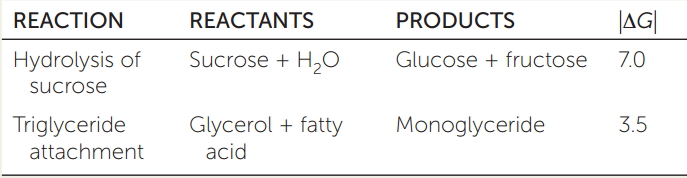
- For each reaction, would you expect ΔG to be positive or negative?
- Which reactions will be spontaneous? Explain your answer.
Why is the search for water important in the search for life?
ANSWER You have seen throughout this chapter that water is essential for the chemistry of life. Water is composed of two of the most abundant elements (Concept 2.1). Water is a polar molecule (Concept 2.2), which allows biologically important polar molecules such as monosaccharides (Concept 2.3) to dissolve in water. Because they are hydrophobic, lipids interact with water to form important biological structures (Concept 2.4). Water molecules participate directly in the formation and breakdown of polymers (Concept 2.2). In short, all of the processes of life as we know it require water.
In the opening essay of this chapter, we described recent evidence for the presence of water on other bodies in our solar system. Could this water harbor life, now or in the past? One way to investigate this possibility is to study how life on Earth may have originated in an aqueous environment. Geological evidence suggests that Earth was formed about 4.5 billion years ago and that life arose about 3.8 billion years ago. During the time when life originated, there was apparently little oxygen gas (O2) in the atmosphere. In the 1950s, Stanley Miller and Harold Urey at the University of Chicago set up an experimental “atmosphere” containing various gases thought to be present in Earth’s early atmosphere. Among them were ammonia (NH3), hydrogen (H2), methane (CH4), and water vapor (H2O). Miller and Urey passed an electric spark over the mixture to simulate lightning, providing a source of energy for covalent bond formation. Then they cooled the system so the gases would condense and collect in a watery solution, or “ocean” (FIGURE 2.16). Note that water was essential for this experiment as a source of oxygen atoms.
Investigation
HYPOTHESIS
Organic chemical compounds can be generated under conditions similar to those that existed in the atmosphere of primitive Earth.
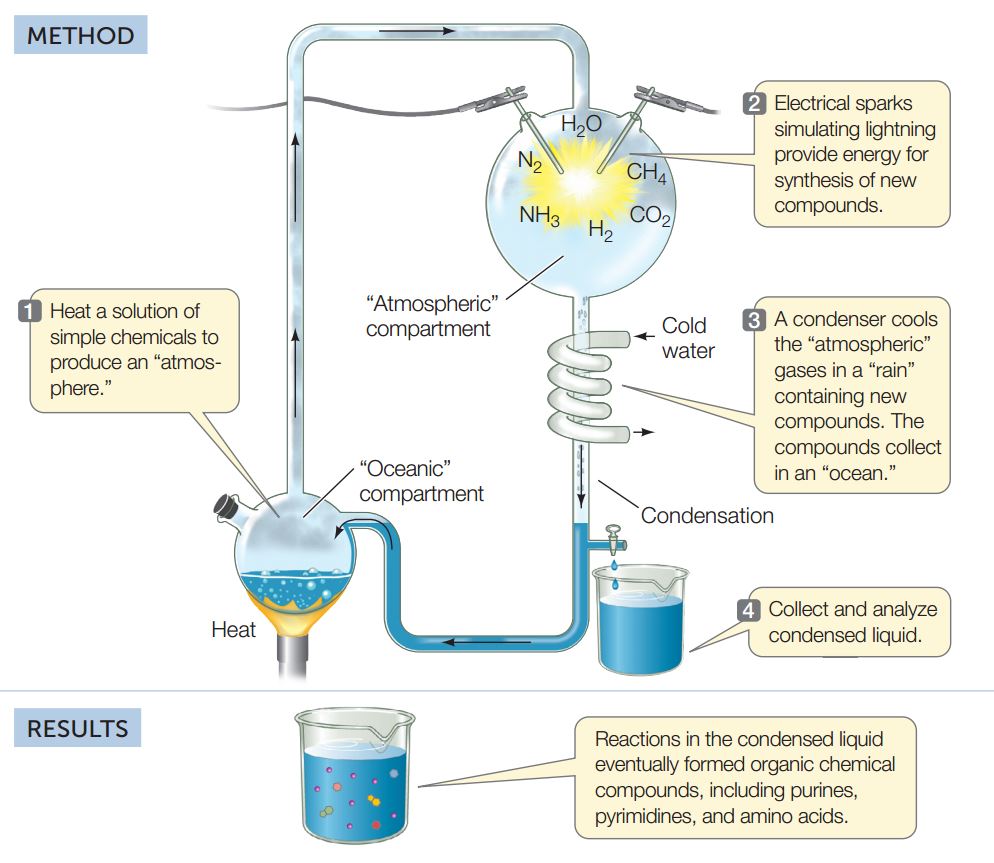
CONCLUSION
The chemical building blocks of life could have been generated in the probable atmosphere of early Earth.
ANALYZE THE DATA
The following data show the amount of energy impinging on Earth in different forms.
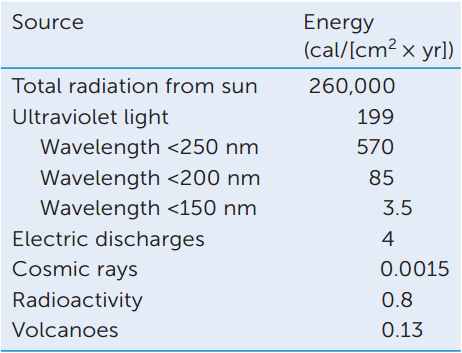
- Only a small fraction of the sun’s energy is ultraviolet light (less than 250 nm). What is the rest of the solar energy?
- The molecules CH4, H2O, NH3, and CO2 absorb light at wavelengths less than 200 nm. What fraction of total solar radiation is in this range?
- Instead of electric discharges, what other sources of energy could be used in these experiments?
aS. L. Miller and H. C. Urey. 1959. Science 130: 245–251.
After several days of continuous operation, the system contained numerous complex molecules, including amino acids, the building blocks of proteins. In later experiments the researchers added other gases, such as carbon dioxide (CO2), nitrogen (N2), and sulfur dioxide (SO2). This resulted in the formation of functional groups such as carboxylic acids, fatty acids, and many three- to six-carbon sugars. Taken together, these data suggest a plausible mechanism for the formation of life’s chemicals in the aqueous environment of early Earth.
35
36