CONCEPT3.3 Some Proteins Act as Enzymes to Speed up Biochemical Reactions
In Chapter 2 we introduced the concepts of biological energetics. We showed that some metabolic reactions are exergonic and some are endergonic, and that biochemistry obeys the laws of thermodynamics (see Figures 2.14 and 2.15). Knowing whether energy is supplied or released in a particular reaction tells us whether the reaction can occur in a living system. But it does not tell us how fast the reaction will occur.
Living systems depend on reactions that occur spontaneously. But without help, most of these reactions would proceed at such slow rates that an organism could not survive. The role of a catalyst is to speed up a reaction without itself being permanently altered. A catalyst does not cause a reaction to occur, but it increases the rate of the reaction. This is an important point: No catalyst makes a reaction occur that would not proceed without it.
Biological catalysts are called enzymes; for example, the synthesis of prostaglandin (see the opening story) is catalyzed by an enzyme (cyclooxygenase). Most enzymes are proteins, but a few important enzymes are RNA molecules called ribozymes. An enzyme can bind the reactants in a chemical reaction and participate in the reaction itself. However, this participation does not permanently change the enzyme. At the end of the reaction, the enzyme is unchanged and available to catalyze additional, similar reactions.
An energy barrier must be overcome to speed up a reaction
An exergonic reaction releases free energy (G), which is the amount of energy in a system that is available to do work. For example, the free energy released in an exergonic reaction can be used by the cell to drive an endergonic reaction, or it can be converted to mechanical energy for movement (see Figure 6.1). But without a catalyst, a reaction will usually take place very slowly. This is because there is an energy barrier between the reactants and the products. Think about the hydrolysis of sucrose, which we described in Concept 2.5.
50
Sucrose + H2O → glucose + fructose
In humans, this reaction is part of the process of digestion. The reaction is exergonic, but even if water is abundant, the sucrose molecule will only rarely bind the H atom and the —OH group in the water molecule at the appropriate locations to break the covalent bond between the glucose and fructose—unless there is an input of energy to initiate the reaction. Such an input of energy will place the sucrose into a reactive mode called the transition state. The energy input required for sucrose to reach this state is called the activation energy (Ea). Once the transition state is reached, the reaction can proceed spontaneously with a release of free energy (ΔG is negative) (FIGURE 3.12A). The image of a ball rolling over a bump and then down a hill helps illustrate these concepts (FIGURE 3.12B).
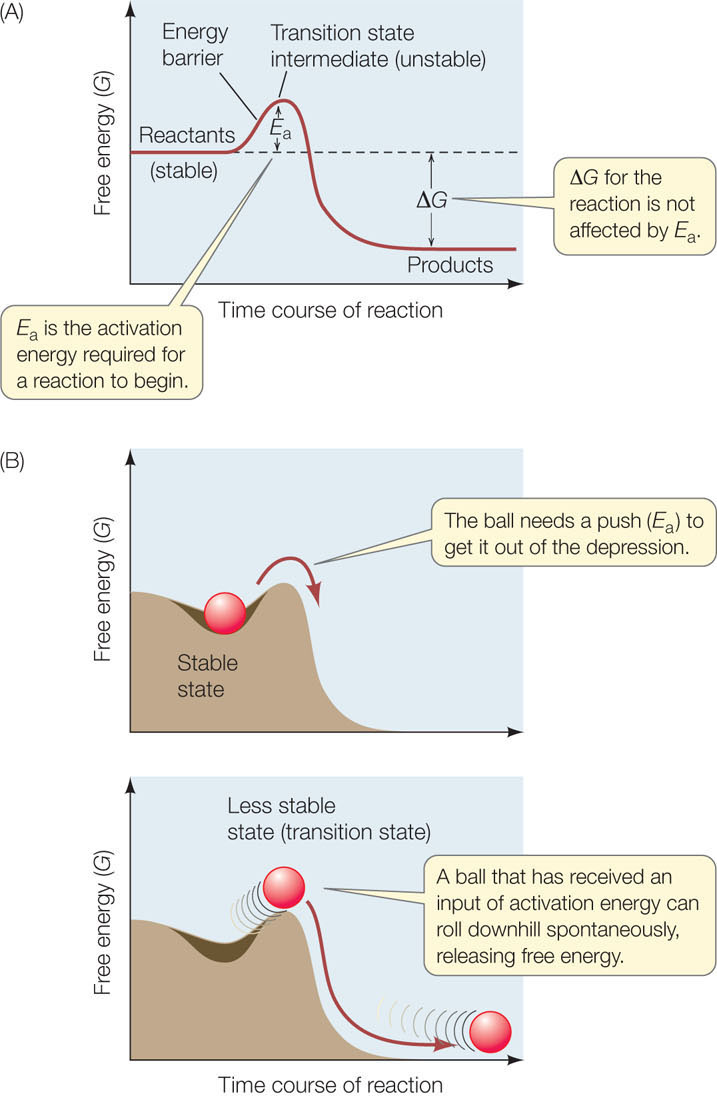
Where does the activation energy come from? In any collection of reactants at room or body temperature, the molecules are moving around. Recall from Chapter 2 that the energy the molecules possess due to this motion is called kinetic energy. A few molecules are moving fast enough that their kinetic energy can overcome the energy barrier; they enter the transition state and react. So the reaction takes place—but very slowly. If the system is heated, all the reactant molecules have more kinetic energy, and the reaction speeds up. You have probably used this technique in the chemistry laboratory.
Adding enough heat to increase the average kinetic energy of the molecules would not work in a living system, however. Such a nonspecific approach would accelerate all reactions, including destructive ones such as the denaturation of proteins.
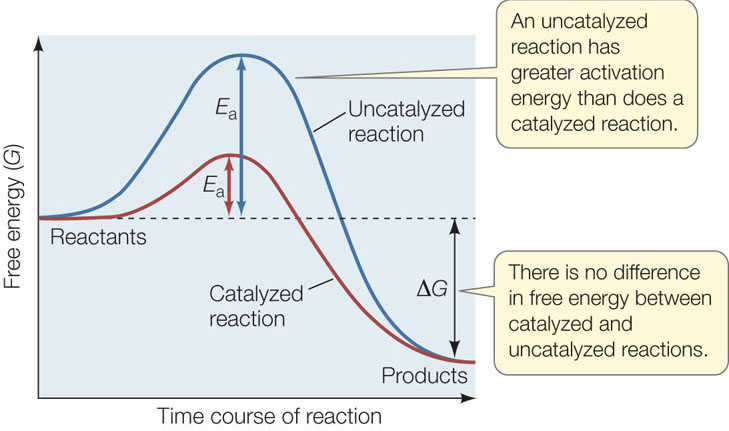
An enzyme lowers the activation energy for a reaction by enabling the reactants to come together and react more easily; the reactants need lower amounts of kinetic energy to enter their transition states (FIGURE 3.13). In this way, an enzyme can change the rate of a reaction substantially. For example, if a molecule of sucrose just sits in solution, hydrolysis may take hundreds of years. But with the enzyme sucrase present, the same reaction occurs in 1 second! Typically, an enzyme-catalyzed reaction proceeds 103 to 108 times faster than the uncatalyzed reaction, and the enzyme converts 100 to 1,000 substrate molecules into product per second.
51
Enzymes bind specific reactants at their active sites
Catalysts increase the rates of chemical reactions. Most nonbiological catalysts are nonspecific. For example, powdered platinum catalyzes virtually any reaction in which molecular hydrogen (H2) is a reactant. In contrast, most biological catalysts are highly specific. An enzyme usually recognizes and binds to only one or a few closely related reactants, and it catalyzes only a single chemical reaction.
In an enzyme-catalyzed reaction, the reactants are called substrates. Substrate molecules bind to a particular site on the enzyme, called the active site, where catalysis takes place (FIGURE 3.14). The specificity of an enzyme results from the exact three-dimensional shape (also called conformation) and chemical properties of its active site. Only a narrow range of substrates, with specific shapes, functional groups, and chemical properties, can fit properly and bind to the active site. The names of enzymes reflect their functions and often end with the suffix “ase.” For example, the enzyme sucrase catalyzes the hydrolysis of sucrose, and we write the reaction as follows:
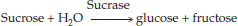
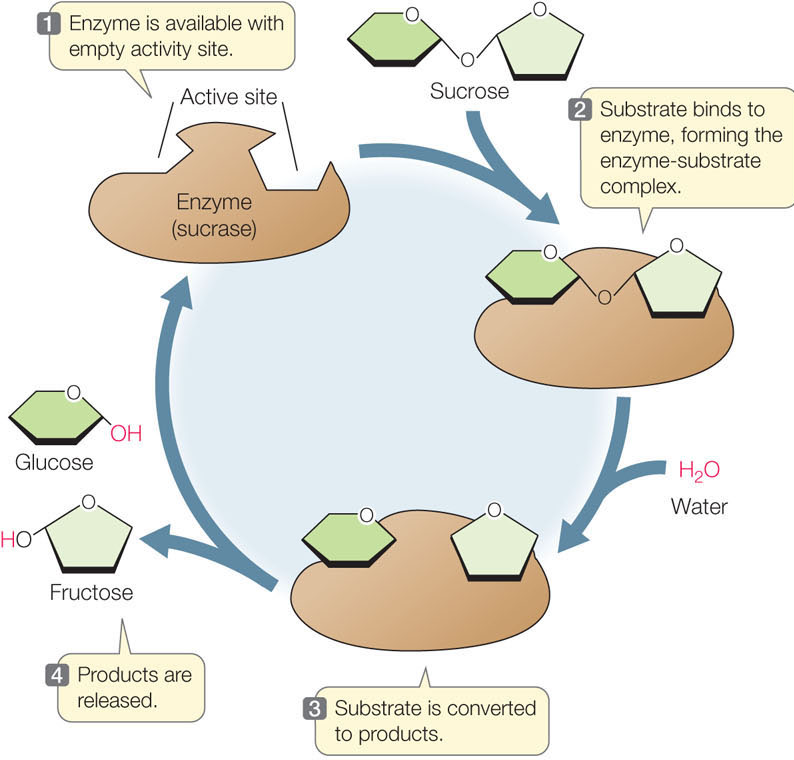
The binding of a substrate (S) to the active site of an enzyme (E) produces an enzyme–substrate complex (ES) that is held together by one or more means, such as hydrogen bonding, ionic attraction, or temporary covalent bonding. The enzyme–substrate complex gives rise to product (P) and free enzyme:
E + S → ES → E + P
(As we have seen in the case of sucrase, a single enzyme-catalyzed reaction may involve multiple substrates and/or products.) The free enzyme (E) is in the same chemical form at the end of the reaction as at the beginning. While bound to the substrate(s), it may change chemically, but by the end of the reaction it has been restored to its initial form and is ready to catalyze the same reaction again (see Figure 3.14).
How Enzymes Work
During and after the formation of the enzyme–substrate complex, chemical interactions occur. These interactions contribute directly to the breaking of old bonds and the formation of new ones. In catalyzing a reaction, an enzyme may use one or more mechanisms:
- Inducing strain: Once the substrate has bound to the active site, the enzyme causes bonds in the substrate to stretch, putting it in an unstable transition state:
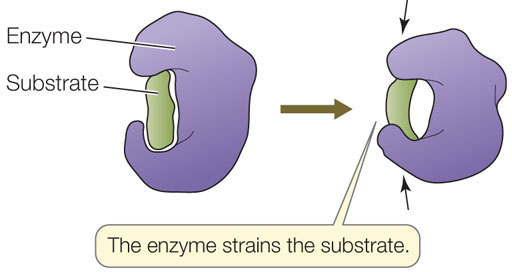
- Substrate orientation: When free in solution, substrates are moving from place to place randomly while at the same time vibrating, rotating, and tumbling. They only rarely have the proper orientation to react when they collide. The enzyme lowers the activation energy needed to start the reaction, by bringing together specific atoms so that bonds can form.
- Adding chemical groups: The side chains (R groups) of an enzyme’s amino acids may be directly involved in the reaction. For example, in acid–base catalysis, the acidic or basic side chains of the amino acids in the active site transfer H+ ions to or from the substrate, destabilizing a covalent bond in the substrate and permitting the bond to break.
The active site is usually only a small part of the enzyme protein. But its three-dimensional structure is so specific that it binds only one or a few related substrates. The binding of the substrate to the active site depends on the same relatively weak forces that maintain the tertiary structure of the enzyme: hydrogen bonds, the attraction and repulsion of charged groups, and hydrophobic interactions. Scientists used to think of substrate binding as being similar to a lock and key fitting together. Actually, for most enzymes and substrates the relationship is more like a baseball and a catcher’s mitt: the substrate first binds, and then the active site changes slightly to make the binding tight. FIGURE 3.15 illustrates this “induced fit” phenomenon. (We introduced the concept of protein structure changes earlier; see Figure 3.11.)
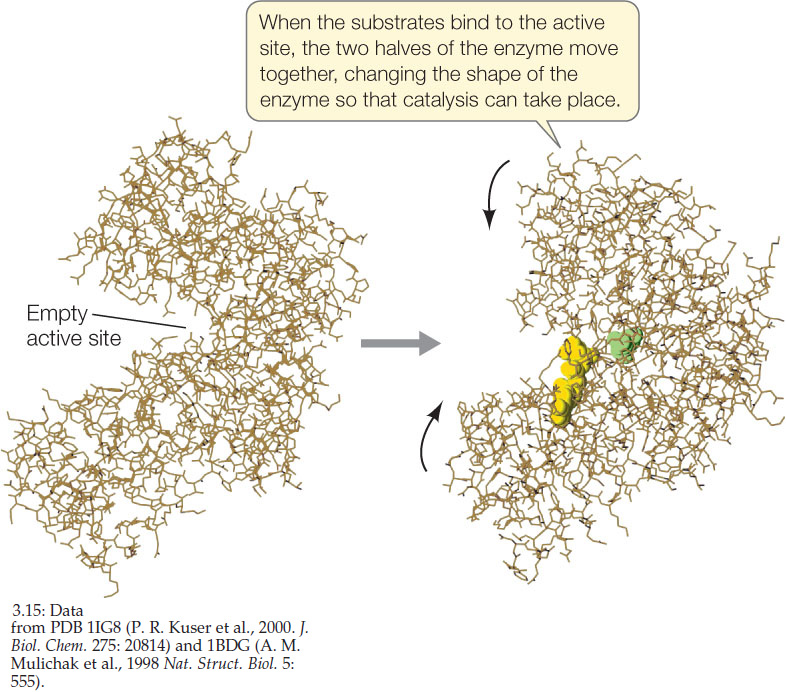
Induced fit at least partly explains why enzymes are so large. The rest of the macromolecule has at least three roles:
52
- It provides a framework so the amino acids of the active site are properly positioned in relation to the substrate(s).
- It participates in the changes in protein shape and structure that result in induced fit.
- It provides binding sites for regulatory molecules (as we will discuss in Concept 3.4).
Nonprotein Partners for Enzymes
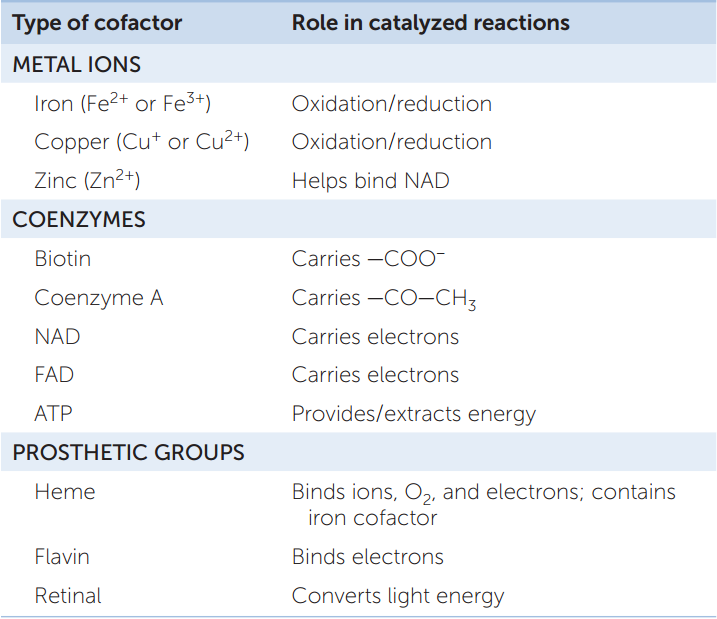
Some enzymes require ions or other molecules in order to function. These molecules are referred to as cofactors, and they can be grouped into three categories (TABLE 3.3):
- Metal ions such as copper, zinc, and iron bind to certain enzymes and participate in the enzyme-catalyzed reactions. For example, the cofactor zinc binds to the enzyme alcohol dehydrogenase, which catalyzes the breakdown of toxic alcohol.
- A coenzyme is a relatively small, carbon-containing (organic) molecule that is required for the action of one or more enzymes. It binds to the active site of the enzyme, adds or removes a chemical group from the substrate, and then separates from the enzyme to participate in other reactions. A coenzyme differs from a substrate in that it can participate in many different reactions with different enzymes.
- Prosthetic groups are organic molecules that are permanently bound to their enzymes. An example is a flavin nucleotide, which binds to succinate dehydrogenase, an important enzyme in energy metabolism.
Rate of Reaction
The rate of an uncatalyzed reaction is directly proportional to the concentration of the substrate. The higher the concentration, the more reactions per unit of time. As we have seen, the addition of the appropriate enzyme speeds up the reaction, but it also changes the shape of the plot of rate versus substrate concentration (FIGURE 3.16). For a given concentration of enzyme, the rate of the enzyme-catalyzed reaction initially increases as the substrate concentration increases from zero, but then it levels off.
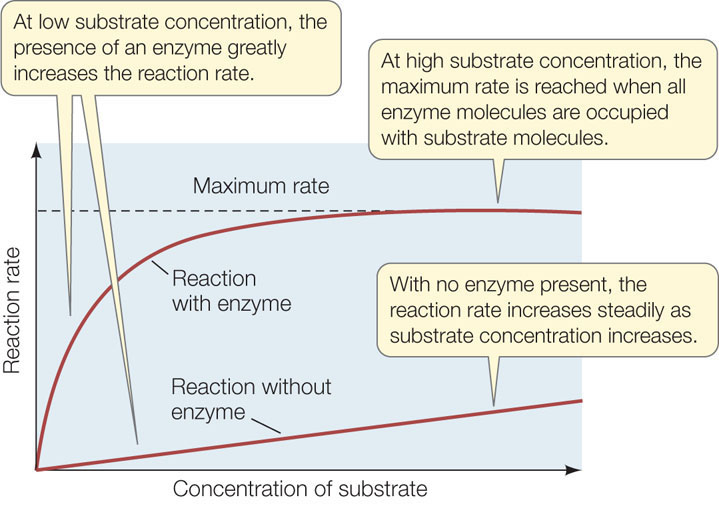
Why does this happen? The concentration of an enzyme is usually much lower than that of its substrate and does not change as substrate concentration changes. When all the enzyme molecules are bound to substrate molecules, the enzyme is working at its maximum rate. Under these conditions the active sites are said to be saturated.
The maximum rate of a catalyzed reaction can be used to measure how efficient the enzyme is—that is, how many molecules of substrate are converted into product by an individual enzyme molecule per unit of time, when there is an excess of substrate present. This turnover number ranges from 1 molecule every second for sucrase to an amazing 40 million molecules per second for the liver enzyme catalase.
CHECKpointCONCEPT3.3
- Explain how the structure of an enzyme makes that enzyme specific.
- What is activation energy? How does an enzyme lower the activation energy needed to start a reaction?
- Compare coenzymes with substrates. How do they work together in enzyme catalysis?
- Compare the state of an enzyme active site at a low substrate concentration and at a high substrate concentration. How does this affect the rate of the reaction?
53
Now that you understand more about how enzymes function, let’s see how different enzymes work in the metabolism of living organisms.