CONCEPT10.5 Proteins Are Modified after Translation
The site of a polypeptide’s function in the cell may be far away from its point of synthesis at the ribosome. This is especially true for eukaryotes, where a polypeptide may be moved into an organelle. Furthermore, polypeptides are often modified by the addition of new chemical groups that contribute to the function of the mature protein. In this section we examine these posttranslational aspects of protein synthesis.
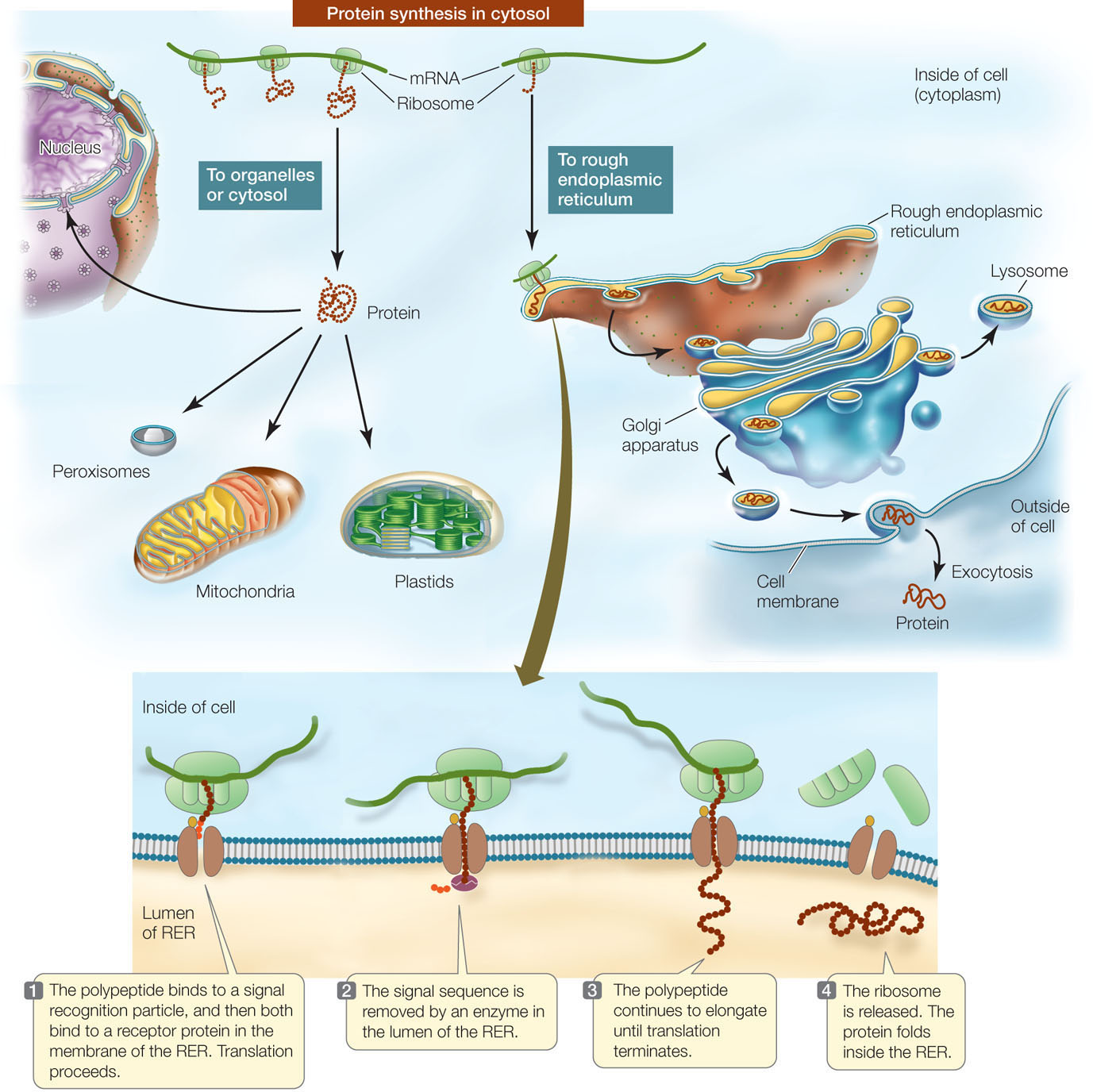
Signal sequences in proteins direct them to their cellular destinations
Protein synthesis always begins on free ribosomes floating in the cytoplasm, and the “default” location for a protein is the cytosol. As the polypeptide chain emerges from the ribosome it may simply fold into its three-dimensional shape and perform its cellular role in the cytosol. However, a newly formed polypeptide may contain a signal sequence (or signal peptide)—a short stretch of amino acids that indicates where in the cell the polypeptide belongs. Proteins destined for different locations have different signals.
In the absence of a signal sequence, the protein will remain in the same cellular compartment where it was synthesized. Some proteins, however, contain signal sequences that “target” them to the nucleus, mitochondria, plastids, or peroxisomes (FIGURE 10.19, LEFT). A signal sequence binds to a specific receptor protein at the surface of the organelle. Once it has bound, a channel forms in the organelle membrane, allowing the targeted protein to move into the organelle. For example, here is a nuclear localization signal (NLS):
212
-Pro-Pro-Lys-Lys-Lys-Arg-Lys-Val-
The function of the NLS was established using experiments like the one illustrated in FIGURE 10.20. Proteins with or without this peptide were introduced into cells and then located by labeling the proteins with fluorescent dyes. Only proteins with the nuclear localization signal were found in the nucleus.
Investigation
HYPOTHESIS
A nuclear localization signal is necessary for importing a protein into the cell nucleus.
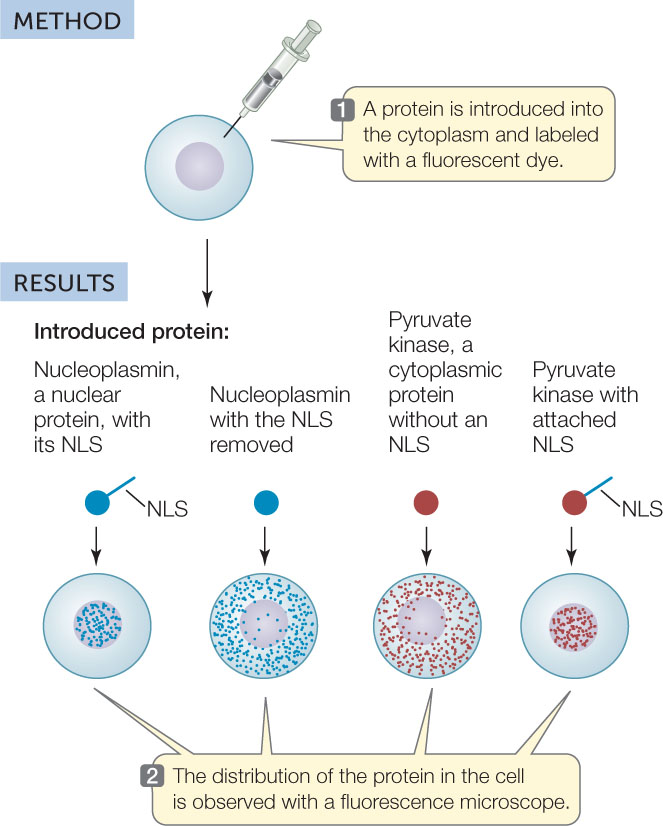
CONCLUSION
An NLS is essential for nuclear protein import and will direct a normally cytoplasmic protein to the nucleus.
a C. Dingwall et al. 1988. Journal of Cell Biology 107: 841-849.
If a polypeptide carries a particular signal sequence of five to ten hydrophobic amino acids at its N terminus, it will be directed to the rough endoplasmic reticulum (RER) for further processing (FIGURE 10.19, RIGHT AND BOTTOM). Translation will pause, and the ribosome will bind to a receptor at the RER membrane. Once the polypeptide–ribosome complex is bound, translation will resume, and as elongation continues, the protein will traverse the RER membrane. Such proteins may be retained in the lumen (the inside) or membrane of the RER, or they may move elsewhere within the endomembrane system (Golgi apparatus, lysosomes, and cell membrane). If the proteins lack specific signals for destinations within the endomembrane system, they are usually secreted from the cell via vesicles that fuse with the cell membrane.
LINK
The endomembrane system and its functions are described in Concept 4.3
Many proteins are modified after translation
Most mature proteins are not identical to the polypeptide chains that are translated from mRNA on the ribosomes. Instead, most polypeptides are modified in any of a number of ways after translation (FIGURE 10.21). These modifications are essential to the final functioning of the protein.
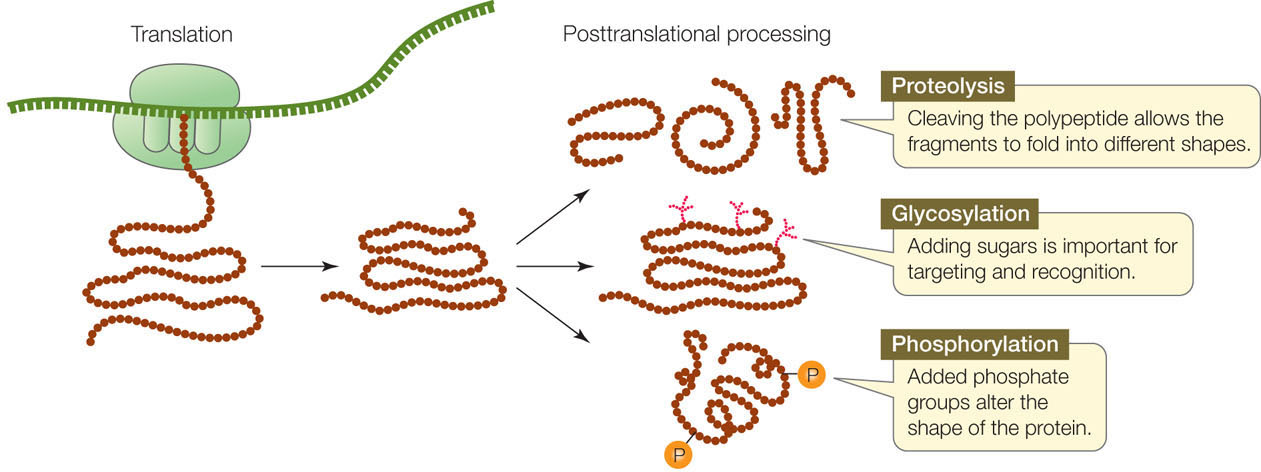
- Proteolysis is the cutting of a polypeptide chain. For example, the ER signal sequence is cut off from the growing polypeptide chain as it enters the ER. Some mature proteins are actually made from polyproteins—long polypeptides containing the primary sequences of multiple distinct proteins—that are cut into final products by enzymes called proteases. Proteases are essential to some viruses, including human immunodeficiency virus (HIV), because the large viral polyprotein cannot fold properly unless it is cut. Certain drugs used to treat acquired immune deficiency syndrome (AIDS) work by inhibiting the HIV protease, thereby preventing the formation of proteins needed for viral reproduction.
- Glycosylation is the addition of carbohydrates to proteins to form glycoproteins. In both the ER and the Golgi apparatus, resident enzymes catalyze the addition of various oligosaccharides (short chains of monosaccharides; see Concept 2.3) to certain amino acid R groups on proteins. One such type of “sugar coating” is essential for directing proteins to lysosomes. Other types are important for protein conformation and for recognition functions at the cell surface. As we noted in Chapter 8, different chains of sugars added to red blood cell proteins determine an individual’s blood type. In many cases the attached oligosaccharides help stabilize proteins, such as those in the extracellular matrix, and those in the storage vacuoles of plants.
- Phosphorylation is the addition of phosphate groups to proteins and is catalyzed by protein kinases. The charged phosphate groups change the conformation of the protein, often exposing the active site of an enzyme or the binding site for another protein. Phosphorylation is especially important in cell signaling (see Concepts 5.5 and 5.6).
213
CHECKpointCONCEPT10.5
- Describe how signal sequences determine where a protein will go after it is made.
- What are some ways in which posttranslational modifications alter protein structure and function?
- Describe an experiment you would perform to test a proposed chloroplast-targeting signal sequence. Be specific about the type of cell and the proteins you would use. Describe the results you would expect if the sequence is indeed a chloroplast-targeting signal.
How do antibiotics target bacterial protein synthesis?
214