CONCEPT11.4 Eukaryotic Gene Expression Can Be Regulated after Transcription
Gene expression involves transcription and then translation. So far we have described how eukaryotic gene expression is regulated at the transcriptional level. But as Figure 11.1 shows, there are many points at which regulation can occur after the initial gene transcript is made.
Different mRNAs can be made from the same gene by alternative splicing
Most primary mRNA transcripts in eukaryotes contain several introns (see Figure 10.6). We have seen how the splicing mechanism recognizes the boundaries between exons and introns. What would happen if the β-globin pre-mRNA, which has two introns, were spliced from the start of the first intron to the end of the second? The middle exon would be spliced out along with the two introns. An entirely new protein (certainly not a β-globin) would be made, and the functions of normal β-globin would be lost. Such alternative splicing can be a deliberate mechanism for generating a family of different proteins with different activities and functions from a single gene (FIGURE 11.16).
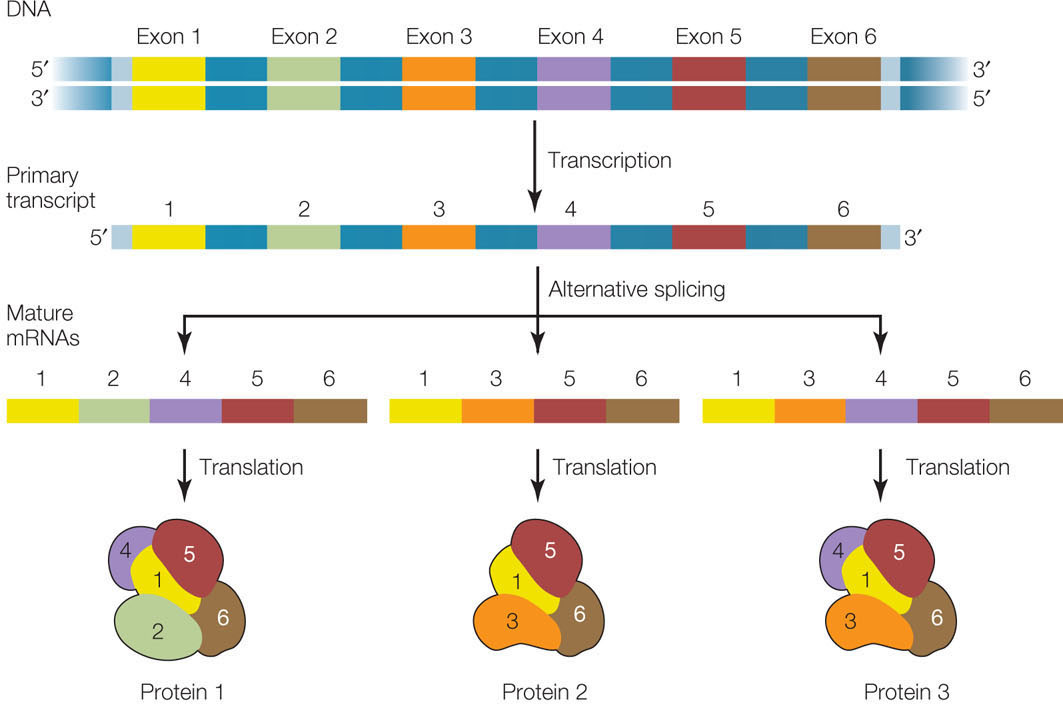
Two examples of this mechanism are found in HIV and in the fruit fly (Drosophila):
- The HIV genome (see Figure 11.12) encodes nine proteins but is transcribed as a single pre-mRNA. Most of the nine proteins are then generated by alternative splicing of this pre-mRNA.
- In Drosophila, sex is determined by the Sxl gene. This gene has four exons, which we will designate 1, 2, 3, and 4. In the female embryo, splicing generates two active forms of the Sxl protein, containing exons 1 and 2, and 1, 2, and 4. However, in the male embryo, the protein contains all four exons (1, 2, 3, and 4) and is inactive.
Before the human genome was sequenced, most scientists estimated that they would find between 80,000 and 150,000 protein-coding genes. You can imagine their surprise when the actual sequence revealed only about 21,000 genes! In fact, there are many more human mRNAs than there are human genes, and most of this variation comes from alternative splicing. Indeed, recent surveys show that more than 80 percent of all human genes are alternatively spliced.
Alternative splicing may be a key to the differences in levels of complexity among organisms. For example, although humans and chimpanzees have similar-sized genomes, there is more alternative splicing in the human brain than in the brain of a chimpanzee.
MicroRNAs are important regulators of gene expression
As we will discuss in Concept 12.3, only a small fraction of the genome in most plants and animals codes for proteins. Some of the genome encodes ribosomal RNA and transfer RNAs, but until recently biologists thought that the rest of the genome was not transcribed; some even called it “junk.” Recent investigations, however, have shown that some of these noncoding regions are transcribed into tiny RNA molecules called microRNA (miRNA).
230
The first miRNA sequences were found in the worm Caenorhabditis elegans. This model organism, which has been studied extensively by developmental biologists, goes through several larval stages. Victor Ambros at the University of Massachusetts found lin mutations (named for abnormal cell lineage) in two genes that had different effects on progress through these stages:
- lin-14 mutations cause the larvae to skip the first stage and go straight to the second stage. Thus the gene’s normal role is to facilitate events of the first larval stage.
- lin-4 mutations cause certain cells in later larval stages to repeat a pattern of development normally observed in the first larval stage. It is as if the cells were stuck in that first stage. So the normal role of this gene is to negatively regulate lin-14, turning off its expression so the cells can progress to the next stage.
Not surprisingly, further investigation showed that lin-14 encodes a transcription factor that affects the transcription of genes involved in larval cell progression. It was originally expected that lin-4, the negative regulator, would encode a protein that downregulates genes activated by the LIN-14 protein. But this turned out to be incorrect. Instead, lin-4 encodes a 22-base miRNA that inhibits lin-14 expression posttranscriptionally by binding to its mRNA.
Thousands of miRNAs in a variety of eukaryotes have now been described. Each miRNA is about 22 nucleotides long and usually has dozens of mRNA targets. MicroRNAs are transcribed as longer precursors that fold into double-stranded RNA molecules, which then are processed through a series of steps into single-stranded miRNAs. A protein complex guides the miRNA to its target mRNA, where translation is inhibited and the mRNA is degraded (FIGURE 11.17). The remarkable conservation of this gene-silencing mechanism, which is found in most eukaryotes, indicates that it is evolutionarily ancient and biologically important.
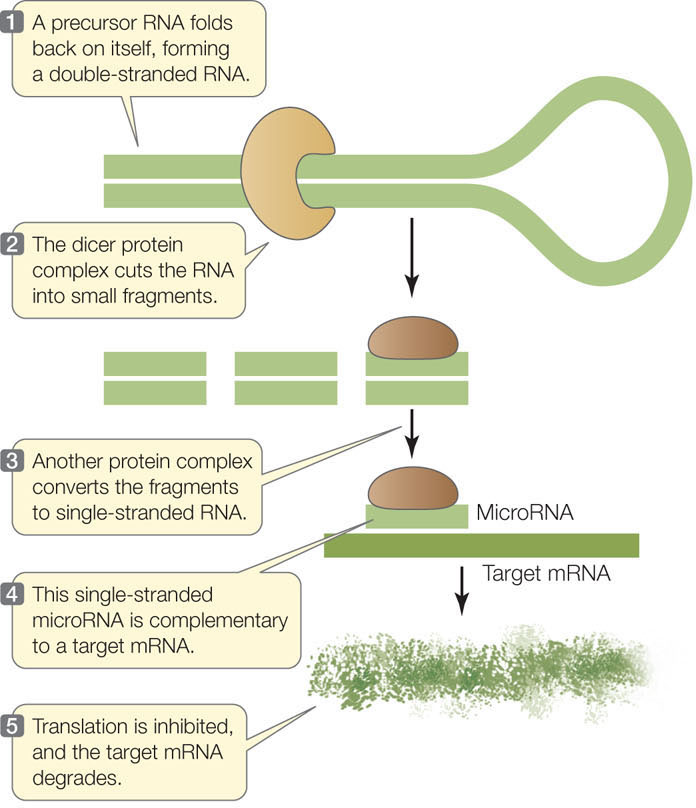
Translation of mRNA can be regulated
The amount of a protein in a cell is not determined simply by the amount of its mRNA. For example, in yeast cells only about a third of the genes show clear correlations in the amounts of mRNA and protein; in these cases, more mRNA leads to more protein. For two-thirds of the genes there is no apparent relationship between the two—there may be lots of mRNA and little or no protein, or lots of protein and little mRNA. The concentrations of these proteins must therefore be determined by factors acting after the mRNA is made. Cells do this in two major ways: by regulating the translation of mRNA or by altering how long proteins persist in the cell.
There are three known ways in which the translation of mRNA can be regulated:
- Inhibition of translation with miRNAs. This was discussed in the last section (see above).
- Modification of the 5′ cap. As noted in Concept 10.2, an mRNA usually has a chemically modified molecule of guanosine triphosphate (GTP) at its 5′ end. An mRNA that is capped with an unmodified GTP molecule is not translated. For example, stored mRNAs in the egg cells of the tobacco hornworm moth are capped with unmodified GTP molecules and are not translated. After the egg is fertilized, however, the caps are modified, allowing the mRNA to be translated to produce the proteins needed for early embryonic development.
231
- Translational repressor proteins. Such proteins block translation by binding to mRNAs and preventing their attachment to the ribosome. For example, in mammalian cells the rate of translation of the protein ferritin increases rapidly when the level of free iron ions (Fe2+) increases in the cell. Iron is an essential nutrient, but the free ions can be toxic to the cell; ferritin binds the ions and stores them in a safe but accessible form. The amount of ferritin mRNA in the cell remains constant, but when the iron level is low, a translational repressor binds to the ferritin mRNA and prevents its translation. When the iron level rises, some of the excess Fe2+ ions bind to the repressor and alter its three-dimensional structure, causing the repressor to detach from the mRNA and allowing translation to proceed (FIGURE 11.18).
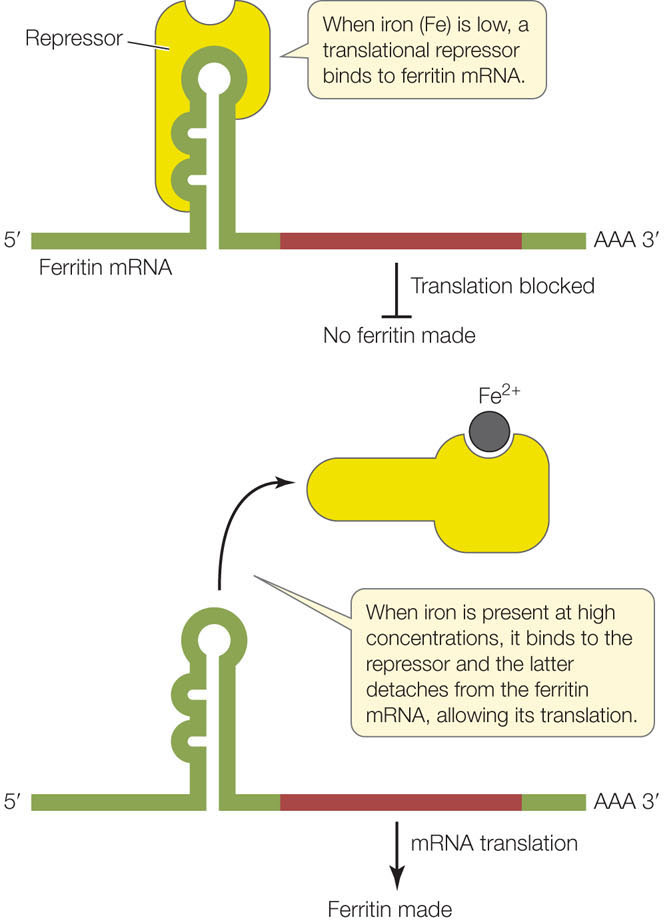 Figure 11.18: A Repressor of Translation Binding of a translational repressor to mRNA blocks the mRNA from associating with the ribosome. The repressor can be removed from the mRNA via allosteric regulation.
Figure 11.18: A Repressor of Translation Binding of a translational repressor to mRNA blocks the mRNA from associating with the ribosome. The repressor can be removed from the mRNA via allosteric regulation.
Protein stability can be regulated
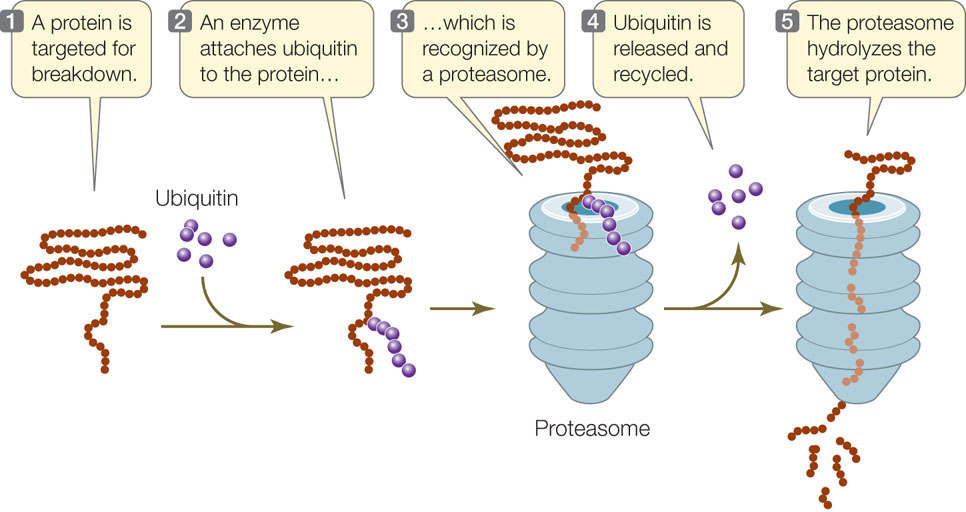
The protein content of any cell at a given time is a function of both protein synthesis and protein degradation. Certain proteins can be targeted for destruction in a chain of events that begins when an enzyme attaches a 76–amino acid protein called ubiquitin (so named because it is ubiquitous, or widespread) to a lysine residue of the protein to be destroyed. Other ubiquitins then attach to the primary one, forming a polyubiquitin chain. The protein–polyubiquitin complex then binds to a huge protein complex called a proteasome (from protease and soma, “body”; FIGURE 11.19). Upon entering the proteasome, the polyubiquitin is removed and ATP energy is used to unfold the target protein. Three different proteases then digest the protein into small peptides and amino acids. You may recall from Chapter 7 that cyclins are proteins that regulate the activities of key enzymes at specific points in the cell cycle. Cyclins must be broken down at just the right time, and this is done by proteasomes.
Eukaryotic gene expression can be regulated transcriptionally and posttranscriptionally
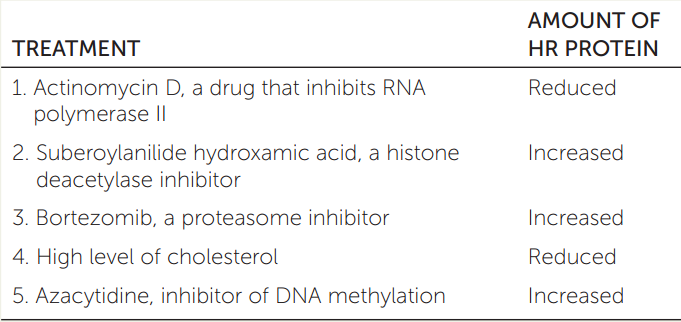
The enzyme HMG CoA reductase (HR) catalyzes an initial step in the synthesis of cholesterol. The table shows the HR levels in liver cells following various treatments. Explain the results of each of the treatments 1–5.
232
CHECKpointCONCEPT11.4
- How can a single gene transcribed into a single pre-mRNA code for several different proteins?
- Compare inhibition of translation by miRNA with inhibition by a repressor.
- You are studying the enzyme protease in germinating seeds. You find that protease activity increases tenfold after treatment of the seeds with a hormone, gibberellic acid. How would you show that this increase is due to:
- release of a translational repressor by the hormone?
- allosteric inhibition of a transcriptional repressor by the hormone?
How does CREB regulate the expression of many genes?
ANSWER CREB is a family of several closely related transcription factors that can either activate or repress gene expression (Concept 11.1). They bind to the cAMP response element (CRE), a short DNA sequence (GACGTCA) that is found in the promoter regions of many genes (Concept 11.2). CREB proteins have a “leucine zipper” structure that consists of two parallel α helices rich in the amino acid leucine, and fingerlike extensions that fit into the major groove of the DNA double helix (FIGURE 11.20).
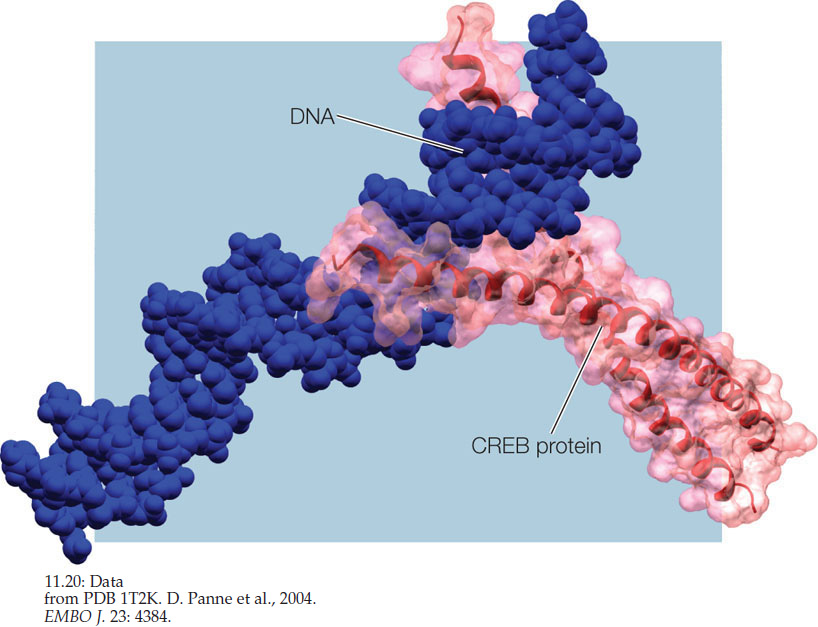
CREB binding and regulation of gene expression are essential for a number of processes in several organs, including the brain. In addition to its role in drug and alcohol addiction (described in the opening story), CREB has been strongly implicated in long-term memory. Animals with mutations that result in a lack of active CREB can learn a maze test, but they don’t remember it later. Similar effects are seen if CREB activity is blocked right after a task is learned. When animals learn a task, imaging studies reveal CRE-containing genes becoming active in the hippocampus, a region of the brain that is involved in long-term memory (see Concept 34.5). Thus CREB provides an insight into the molecular biology of memory, linking learning to the regulation of gene expression.
233